USP 88 In-Vivo Cytotoxicity Testing
What Is Cytotoxicity?
Cytotoxicity refers to molecules and compounds that are poisonous to living cells. Cytotoxins are often chemical but can also be from natural or biological sources. USP 88 testing for in vivo cytotoxicity assays, such as systemic injection, and plastics determination for USP 88 class VI plastics are covered in this article.
What Is Cytotoxicity Testing?
Cytotoxicity testing evaluates the biological reactivity of mammalian cells and tissues to contact with elastomeric plastics, excipients, and other materials that will come in direct or indirect patient contact during medical product use. Thus, sometimes cytotoxicity testing is referred to as biological reactivity testing. Cytotoxicity is significant as it evaluates the biological effects of a sample’s leachable chemicals. The types of cytotoxicity testing to perform for your medical device or product depend upon the final product, the final product’s intended use, and the materials the final product is made of and packaged within.
How Are Cytotoxicity Tests Performed?
Most plastics (polymers) used for injectable, parenteral, and medical products will only require in-vitro cytotoxicity testing covered by USP 87. However, if material components do not meet the requirements of USP 87 direct contact, agar diffusion, and elution testing, in-vivo cytotoxicity testing outlined in USP 88 will be needed. The required in-vivo testing will be implantation, intracutaneous injection, or systemic injection tests. A description of all in-vitro cytotoxicity tests is provided below. For a complete comparison of all benchtop and animal studies for cytotoxicity, please see our article on “In-Vitro USP 87 Vs. In-Vivo USP 88 Cytotoxicity Testing.”

Classification Of Plastics
Plastic polymers evaluated for toxicity fall under six classes. Plastics are classified based on their extractants (solutions in which polymer extracts will be prepared). Plastics are also classified based on the route of administration (e.g., intramuscular, intravenous, etc.). Lastly, plastics are classified by their material properties. These tests are directly related to the intended end-use of the plastics. The extractant(s) choice is based on any solution(s) in the preparations of the plastics they are likely to contact. Table 1 below (a reproduction of Table 1 of USP 88) summarizes six plastic classes based on test material, animal model, dosage, and procedure (route of administration). These classifications summarize which animal tests are needed and how extracts or implant samples should be prepared for various plastics.
In Table 1, the USP 88 testing required for each plastic class (including USP 88 class VI) are indicated by “x” in the appropriate columns. Under the procedure column, A (IP) stands for systemic injection test, B (IC) stands for the intracutaneous test, and C stands for the implantation test. Note that USP 88 class VI plastics have the greatest testing requirements. Most USP 88 class VI elastomers are used in medical and pharmaceutical products like sanitary pumps, tubing, and single-use bags. PTFE seals are a USP 88 class VI product along with Swedish Metric Standard (SMS) 1149 Seals. Polymers that have already undergone USP 88 class VI testing do not necessarily need to be retested. However, modified USP 88 class VI polymers are likely to require in vivo cytoxicity assays.

What Is In-Vivo Cytotoxicity Testing?
In-vivo tests are required in certain instances (such as USP 88 class VI plastics) to determine the systemic biological reactivity of mammalian cells to contact with elastomeric plastics and other polymeric materials. The cytotoxicity tests performed for your medical device or product depend upon the final product’s packaging, intended use, and material construction. Three in-vivo cytotoxicity tests described in USP 88 are used to assess systemic biological reactivity. These tests are implantation, intracutaneous injection, and systemic injection studies.
How Is Implantation Cytotoxicity Testing Performed?
The implantation test is designed to evaluate the reaction of living tissue to polymers by implanting the device or therapeutic product into animal tissue. Material samples must be aseptically prepared and surgically placed under aseptic conditions to ensure accurate implantation results. The factors below should be considered and tested when evaluating the cytotoxicity of implanted polymers.
Factors that can impact the suitability of a material for its intended use are:
- Material composition
- Material processing and cleaning procedures
- Contacting media(s)
- Inks
- Adhesives
- Absorption properties
- Adsorption and permeability of preservatives
- Conditions of storage
The implantation tests are performed by surgically introducing a material sample under evaluation into a test animal. There are two forms of implantation testing, intramuscular implantation in rabbits and subcutaneous implantation in rats. Both implantation studies are described in further detail below.
Intramuscular Implantation In Rabbits
Intramuscular implantation testing uses healthy adult New Zealand rabbits. For intramuscular implantation, test specimens are placed into needles for implantation delivery. Materials with physical characteristics that prevent needle delivery should use the subcutaneous rat implantation model instead.
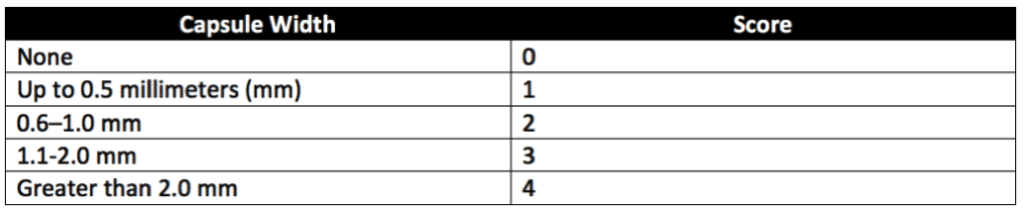
For product implantation, eight strips of the sample material and four USP-approved, high-density polyethylene RS strips are prepared. Each strip should measure not less than ten millimeters by one millimeter. To avoid hurting tissues upon implantation, the edges of the strips should be as smooth as possible. Each strip is implanted using a hypodermic needle (15–19 gauge) with an intravenous point and a sterile trocar. Strips are embedded by inserting the sanitary plastic strips into the hypodermic needle and then using the needle to implant the strips into the rabbits’ muscular tissues. Healthy adult rabbits weighing not less than 2.5 kilograms and with paravertebral muscles large enough for test strip implantation are used. Only paravertebral muscles are used for implantation sites. After the animals are anesthetized, the fur along the implantation areas of each animal is clipped. Then the implantation sites are cleaned. Next, four sample strips are implanted into the paravertebral muscle on one side of each of the two rabbits’ spines. Two strips of high-density polyethylene RS are embedded in the opposite paravertebral muscle of each animal as negative controls. Animals are exposed to the implanted strips for at least 5-days (120-hours) before tissue assessment. The tissue area surrounding the center of each implant strip is examined microscopically. Sample and control implant sites are assessed for hemorrhage, necrosis, discolorations, and infections. All observations are recorded, and encapsulation sites are measured to the nearest 0.1 millimeters. The scoring system in Table 2 below is used for tissue assessment. After all tissue scores are taken, differences between the average scores for the sample and control sites are calculated. Samples pass the implantation test if the calculated difference does not exceed 1.0 or if the difference between the sample and control mean scores for more than one of the four implant sites does not exceed 1 for any implanted animal.
Subcutaneous Implantation In Rats
For subcutaneous implantation in rats, ten samples and ten controls (USP high-density polyethylene RS) are prepared for the experiment. To avoid hurting tissues upon implantation, the edges of the sample and control strips should be as smooth as possible. Five healthy albino rats weighing 225–350 grams are used for testing. Implantation sites along the rat’s spinal columns are prepared similarly to the implantation sites for rabbits. The rats are anesthetized via AAALAC guidelines. After the initial incision, blunt dissection is used to separate the fascia connecting skin to muscle to form a pocket underneath the skin lateral to each side of the incision. A sterile sample is inserted into each tissue pocket, and the incision closed with wound clips or sutures. Two test samples and two control samples are implanted in each of the five rats. The animals are kept for at least seven days following sample implantation before tissues are assessed.
Similar to implantation in rabbits, the area of the tissue surrounding the center of each implant strip is examined microscopically. Sample and control implant sites are assessed for necrosis, hemorrhage, discolorations, and infections. All tissue observations are recorded, and encapsulation sites are measured. The scoring system in Table 2 (Table 6 of USP 88) is used for skin reaction assessment following surgical implantation in animals. After all tissue scores are taken, differences between the average scores for the sample and control sites are calculated. The implantation test is passed if the difference does not exceed 1.0 or if the difference between the sample and control mean scores for more than one of the four implant sites does not exceed 1 for any implanted animal.
What Is In-Vivo Systemic Injection Testing For Cytotoxicity?
A systemic injection is an injection into the circulatory system. Systemic injection testing determines the local biological responses of animals (mice) to plastic extracts injected into the bloodstream. Systemic injection testing and intracutaneous testing may be performed using the same extracts. Extracts are prepared depending on the heat resistance of the material being assessed. Thus, extracts are prepared at 50°, 70°, or 121°C.
Extracts are often classified by the type of plastic (Table 1) and extract temperature. For example, a class IV plastic extracted at 121° would be referred to as IV-121°. Plastics used for oral or topical products containers do not apply for Table 1 classification. Additionally, Table 1 classifications do not apply to natural elastomers. Natural elastomers are tested in sodium chloride injection and vegetable oils only. Sample sizes for extract preparation are detailed in Table 3 below.

Extraction conditions should not cause physical changes such as fusion or melting of the sample pieces. Fusion or melting of sample pieces would decrease the available surface area and jeopardize the results.
In general, extraction conditions of most importance are:
- Contact of the extracting medium with the available surface area of the plastic
- Extraction temperature
- Extraction time
- Proper extract cooling
- Proper extract agitation
- Proper extract decanting
- Aseptic handling and storage of the extracts following extraction
For edema scoring, the scale described in Table 2 of USP 88 (reproduced as Table 4 below) excludes noninflammatory (mechanical) edema from the blank or extraction fluid. Further, the scale in Table 4 uses the scoring system described in “Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes” (Draize JH et al.). Oil residue at the injection site should not be misinterpreted as edema. You can check oil reside versus edematous tissue by applying gentle pressure to the area. Edematous blanches when gentle pressure is applied.

Systemically injected mice are assessed immediately after injection and at 4, 24, 48, and 72 hours after injection. Each study uses ten mice. All mice injected with polymer extracts must show the same or less reactivity as controls injected with blanks to pass this examination.
The sample does not meet the test requirements if one or more of three situations occur. Situation one is two or more mice die. Situation two is abnormal behavior, such as convulsions or prostration, occurs in two or more mice. The final situation is if a bodyweight loss greater than 2 grams occurs in three or more mice.
Tests are repeated with an additional 10 mice if any animals treated with the sample extract show only slight signs of biological reactivity, and not more than one animal shows gross symptoms of biological reactivity or dies. On the repeat test, all 10 animals treated with the sample extract must show no significant biological reactivity above the blank animals during the observation periods to pass the systemic cytotoxicity testing requirements.
What Is In-Vivo Intracutaneous Testing For Cytotoxicity?
An intracutaneous injection is an injection between the layers of the skin. Intracutaneous testing determines the local biological responses of animals (rabbits or guinea pigs) to plastic extracts injected under the skin. Systemic injection testing and intracutaneous testing may be performed using the same extracts. Extracts are prepared depending on the heat resistance of the material being assessed. Thus, extracts are prepared at either 50°, 70°, or 121°C. Extracts are often classified by the type of plastic (Table 1) and extract temperature. For example, a class IV plastic extracted at 121° would be referred to as IV-121°. Plastics used for containers for oral or topical products do not apply for Table 1 classification. Additionally, Table 1 classifications do not apply to natural elastomers, which are tested in sodium chloride injection and vegetable oils only. Sample sizes for extract preparation are detailed in Table 3 above. Further, the same extract conditions and edema scoring system (Table 4) for systemic injection testing should be used for intracutaneous testing.
Intracutaneous testing determines the local response of an animal (rabbits or guinea pigs) to material extracts. For testing, animals are shaved, and any loose hair is vacuumed away. Care is taken to prevent any mechanical irritation or trauma of the animal’s skin before injection. Each extract is shaken vigorously before injection to ensure an even distribution of the extracted matter. More than one extract from a given material can be used per rabbit or guinea pig (if the test results will not be affected). For each sample, two animals are injected intracutaneously. One side of the animal is injected with the sample extract, and the other side is injected with the negative control (Blank). Table 5 of USP 88 describes this process and is reproduced in Table 5 below. Note that blanks are composed of the media used for extract preparation. Ensure that the media used for blanks has not encountered the sample materials being assessed.

After injection, injection sites are assessed for evidence of any tissue reaction such as erythema, edema, and necrosis using the scaring system in Table 4. All animals are observed at 24, 48, and 72 hours after injection for tissue reaction. The average erythema and edema scores for the extract and blank injection sites are determined at every scoring interval (24, 48, and 72 hours) for each rabbit or guinea pig.
After the 72-hour scoring is complete, all erythema scores plus edema scores are totaled separately for each extract sample and blank. Each total is divided by twelve (two animals × three scoring periods × two scoring categories) to determine the overall mean score for each sample extract versus each corresponding blank. The test requirements are met if the difference between the extract samples’ mean score and the blanks’ mean score (negative controls) is 1.0 or less. The test is repeated with three additional animals if the average reaction to the sample extract is questionably greater than the average reaction to the blank (at any observation period).
What Are The Differences Between In-Vivo Implantation, Intracutaneous Injection, And Systemic Injection Testing?
Unlike systemic injection and intracutaneous testing, implantation testing does not use product extracts for testing procedures. Instead, small pieces of the product or materials are directly implanted into rabbit or rat tissues for implantation testing. Implantable medical devices would likely undergo implantation cytotoxicity testing because implantation testing best models the product’s use in patients. Products in direct contact with the circulatory system or that recirculate blood for a patient would undergo systemic injection testing. Indeed, systemic injection better models the exposure level of the device with patient fluids in this instance. Intracutaneous testing evaluates cytotoxicity reactions at the deepest skin level. Thus, intracutaneous testing might evaluate plastics used in surgical equipment that interact with deep skin tissues for short periods.
Summary
Overall, cytotoxicity testing evaluates the toxicity of the polymeric materials used by medical devices and products. The types of cytotoxicity testing needed for your medical device or product will depend upon the final product’s construction, intended use, and packaging materials. This article compares three cytotoxicity tests in live animals from USP 88. These in-vivo tests are implantation, intracutaneous injection, and systemic injection studies. Only in-vitro (benchtop) testing will be necessary for most medical devices to evaluate cytotoxicity. However, cytotoxicity testing in animals is required upon FDA request or for medical devices that cannot be tested for cytotoxicity via benchtop methods. All in all, ensure you choose a contract testing organization that can support you with appropriate cytotoxicity testing for your unique medical device or product needs.
Ethide Labs is a contract testing organization that specializes in Cytotoxicity Testing. Ethide Labs also offers Microbiology Testing, Bioburden Testing, Bacterial Endotoxin Testing, Ethylene Oxide Residual Testing, Sterility Testing, Environmental Monitoring & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <87> Biological Reactivity Tests, In Vitro. Rockville, MD, USA. 2021. (USPC <87>).
United States Pharmacopeial Convention. <88> Biological Reactivity Tests, In Vivo. Rockville, MD, USA. 2021. (USPC <88>).
Share this in your social networks


