USP 87 In-Vitro Cytotoxicity Testing
What Is Cytotoxicity?
Cytotoxicity refers to molecules and compounds that are poisonous to living cells. Cytotoxins are often chemical but can also be from natural or biological sources. This article covers the in vitro cytotoxicity assays based on USP 87 cytotoxicity tests, such as agar diffusion tests. Also covered are biological reactivity grades and more!
What Is Cytotoxicity Testing?
In vitro cytotoxicity assays evaluate the biological reactivity of mammalian cells and tissues to contact with elastomeric plastics, excipients, and other materials that will come in direct or indirect patient contact during medical product use. Thus, sometimes cytotoxicity testing is referred to as biological reactivity testing. Cytotoxicity is significant as it evaluates the biological effects of a sample’s leachable chemicals. The types of in vitro cytotoxicity assays to perform for your medical device or product depend upon the final product, the final product’s intended use, and the materials the final product is made of and packaged within.
How Are Cytotoxicity Tests Performed?
Most plastics (polymers) used for injectable, parenteral, and medical products will only require in vitro cytotoxicity testing covered by USP 87. However, if material components do not meet the requirements of USP 87 direct contact, agar diffusion, and elution testing, in vivo cytotoxicity assays outlined in USP 88 will be needed. The required in vivo testing will be implantation, intracutaneous injection, or systemic injection tests. A description of all in vitro cytotoxicity tests is provided below. For a complete comparison of all benchtop and animal studies for cytotoxicity, please see our article on “In Vitro USP 87 Vs. In Vivo USP 88 Cytotoxicity Testing.”
In Vitro Cytotoxicity Testing (USP 87)
In vitro tests are required to determine the biological reactivity of mammalian cells to contact with elastomeric plastics and other polymeric materials. Three in vitro cytotoxicity tests are used to assess systemic biological reactivity without the need for in vivo animal testing. These tests are direct contact, agar diffusion, and elution assays.
What Is In-Vitro Direct Contact Testing?
Direct contact in vitro cytotoxicity assays can evaluate nearly all materials. Additionally, sample extraction and testing of a sample’s leachable chemicals can coincide with direct contact testing. Direct contact methods cannot assess very low density or extremely high-density materials that could cause mechanical damage to cultured live cells.
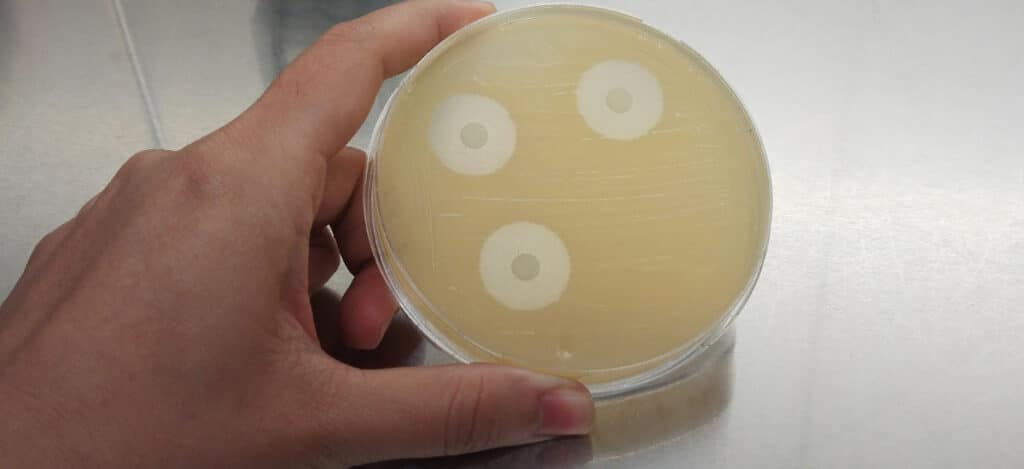
What Is In-Vitro Agar Diffusion Testing?
Agar diffusion tests are beneficial for assessing the cytotoxicity of elastomeric closures. In these tests, the agar layer acts as a cushion. The agar protects the cells from any mechanical damage and allows leachable chemicals to diffuse from the product or packaging samples. The cells are then evaluated to determine the toxicity of the samples. Material extracts can also be assessed for cytotoxicity using the agar diffusion test by applying material extracts to a piece of filter paper.
What Is In-Vitro Elution Testing?
Elution tests are designed for evaluating extracts from plastic materials. Elution tests for cytotoxicity are beneficial for assessing high-density materials and evaluating dose-response in vitro. Elution testing methods allow sample extraction to occur multiple times and under various temperature conditions.
How Are Direct Contact Tests Performed?
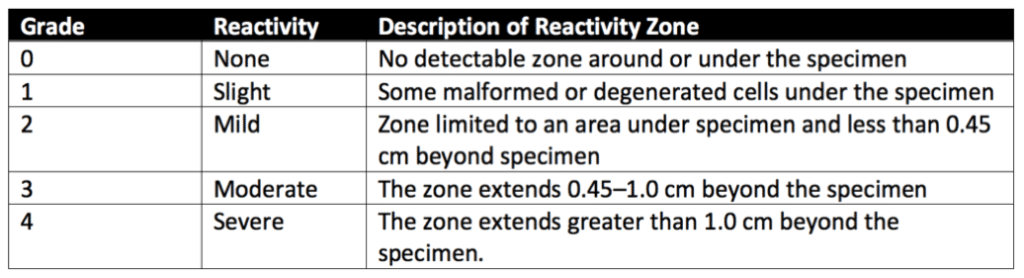
Sample pieces with flat surfaces of at least 100 square millimeters (mm) are prepared for direct contact testing. Polyurethane film with zinc (ZDEC2 or ZDBC) is used as a positive control. Next, L-929 mammalian fibroblast cells are grown in a serum-supplemented minimum essential medium (MEM), and a cell suspension is prepared. Equal amounts of the L-929 cell suspension are added to culture plates with a 35 mm diameter to create a single layer cell culture. After L-929 cells have been cultured to reach the appropriate confluence, the cell culture medium is aspirated from the plates and replaced with a fresh culture medium. Next, a single item (i.e., product sample, positive control, or negative control) is placed in each 35 mm culture dish. All samples and controls are cultured in duplicate or triplicate at 37 ± 1°C in a humidified incubator containing 5 ± 1% carbon dioxide.
After incubation, each sample, positive control, and negative control are examined under a microscope. In some cases, cells are stained to support assessing biological reactivity. The biological reactivity of the cells exposed to the sample or sample extracts is rated on a scale of 0-4 (see Table 1 below for details). The biological reactivity is determined by assessing the nonlethal injury of the cells (cellular degeneration) and any structural defects (malformations) the cells have. The direct contact test is valid if the observed responses to the negative controls are grade 0 and the Positive controls are all at least grade 3. The sample meets the requirements of the direct contact test if the biological responses of the samples are not greater than grade 2.
How Are Agar Diffusion Tests Performed?
For agar diffusion testing, materials or medical device extracts are prepared using a 0.9% sodium chloride injection or a mammalian cell culture media. For samples that aren’t extracts, portions of the test samples with flat surfaces not less than 100 square millimeters (mm) in surface area are used for the agar diffusion assay. Polyurethane film with zinc (ZDEC2 or ZDBC) is used as a positive control.
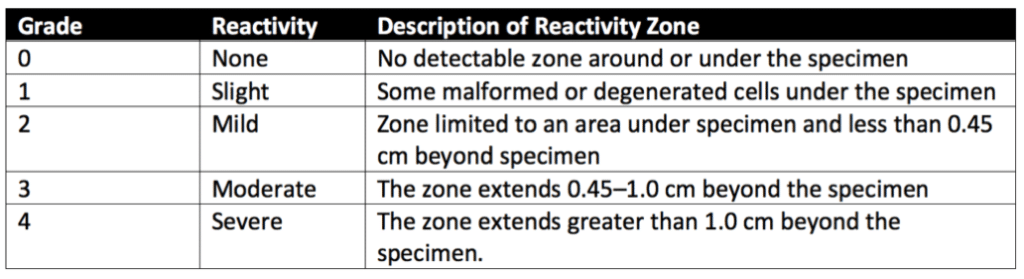
Next, L-929 mammalian fibroblast cells are grown in a serum-supplemented minimum essential medium (MEM) to greater than 80% confluence. The culture medium is then aspirated, and a solution of not more than 2% agar is added to each 60 mm diameter plate. The agar layer must be thin enough to allow the diffusion of leached chemicals from the samples. Finally, samples, positive controls, and negative controls (or their extracts) are added on top of the solidified agar surface. All samples and controls are cultured in duplicate. Then all of the cultures are incubated at 37 ± 1°C in a humidified incubator containing 5 ± 1% of carbon dioxide.
After incubation, cells exposed to samples, positive controls, and negative controls are stained or evaluated without staining under the microscope. The biological reactivity of the cells exposed to the sample or sample extracts is rated on a scale of 0-4 (see Table 2 below for details). The biological reactivity is determined by assessing the nonlethal injury of the cells (cellular degeneration) and any structural defects (malformations) the cells have. The agar diffusion test is valid if the observed responses to the negative controls are grade 0 and the positive controls are all at least grade 3. The sample meets the agar diffusion test’s requirements if the samples’ biological responses are not greater than grade 2.
How Is Elution Testing Performed?
For elution testing, product or material extracts are prepared using a 0.9% sodium chloride injection or mammalian cell culture media. Often 0.1 g of elastomeric materials or 0.2 g of other plastic material is used per milliliter of extraction medium. Polyurethane film with zinc (ZDEC2 or ZDBC) is used as a positive control.
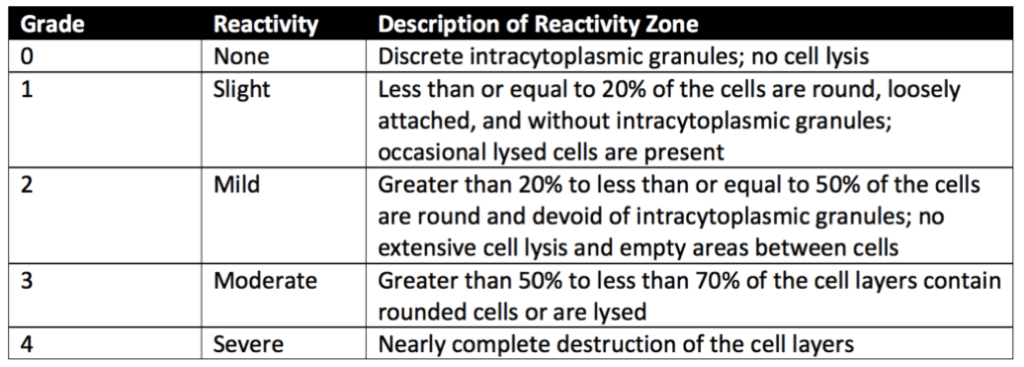
Next, L-929 mammalian fibroblast cells are grown in a serum-supplemented minimum essential medium (MEM), and a cell suspension is prepared. Equal amounts of the L-929 cell suspension are added to culture plates with a 35-millimeter diameter to create a single-layer cell culture. After L-929 cells have been cultured to reach the appropriate confluence, the cell culture medium is aspirated from the plates and replaced with extracts from material samples, positive controls, and negative controls. Extracts from cell culture media remain without dilutions. Extracts prepared with the Sodium Chloride Injection are diluted to a 25% extract concentration with a serum-supplemented cell culture medium. All samples and controls are cultured in duplicate or triplicate at 37 ± 1°C in a humidified incubator containing 5 ± 1% carbon dioxide.
After incubation, cells exposed to samples, positive controls, and negative controls are stained or evaluated without staining under the microscope. The biological reactivity of the cells exposed to the sample or sample extracts is rated on a scale of 0-4 (see Table 3 below for details). The biological reactivity is determined by assessing the nonlethal injury of the cells (cellular degeneration) and any structural defects (malformations) the cells have. The elution test is valid if the observed responses to the negative controls are grade 0 and the positive controls are all at least grade 3. The sample meets the elution test’s requirements if the samples’ biological responses are not greater than grade 2.
What Are The Differences Between In-Vitro Direct Contact, Agar Diffusion, And Elution Testing?
Direct contact tests evaluate medical devices, materials, or packaging sample pieces with flat surfaces not less than 100 square millimeters (mm). In contrast, elution tests assess the cytotoxicity of liquid extracts from sample pieces. Agar diffusion tests are best for evaluating elastomeric closures due to the utilization of an agarose layer for in vitro cytotoxicity assays. For all in vitro test methods, L-929 fibroblast cells are used to evaluate cytotoxicity.
For individual comparisons between benchtop cytotoxicity methods, please see the following articles below:
Summary
Overall, cytotoxicity testing evaluates the toxicity of the polymeric materials used by medical devices and products. This article compares three in vitro cytotoxicity assays from USP 87. These benchtop cytotoxicity tests are direct contact, agar diffusion, and elution testing. Only USP 87 in vitro testing will be necessary to evaluate cytotoxicity in most circumstances. Further, the types of benchtop cytotoxicity testing needed for your medical device or product will depend upon the final product’s construction, intended use, and packaging materials. All in all, ensure you choose a contract testing organization that can support you with appropriate cytotoxicity testing for your unique medical device or product needs.
Ethide Labs is a contract testing organization that specializes in Cytotoxicity Testing. Ethide Labs also offers Microbiology Testing, Bioburden Testing, Bacterial Endotoxin Testing, Ethylene Oxide Residual Testing, Sterility Testing, Environmental Monitoring & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <87> Biological Reactivity Tests, In Vitro. Rockville, MD, USA. 2021. (USPC <87>).
United States Pharmacopeial Convention. <88> Biological Reactivity Tests, In Vivo. Rockville, MD, USA. 2021. (USPC <88>).
Share this in your social networks


