What Is An Elution Test For Cytotoxicity?
What is cytotoxicity testing, and why is it essential for your medical device or product?
Cytotoxicity testing is traditionally a series of in-vitro tests that determine the biological reactivity of mammalian cell cultures to contact with elastomeric plastics and other polymeric materials that will come in direct or indirect patient contact during medical device or product use. An elution test is one of three standard tests for cytotoxicity, the other tests being agar diffusion and direct contact testing. The types of tests to perform for your medical device or product depend upon the final product, the final product’s intended use, and the materials the final product is made of and packaged within. For more information on cytotoxicity tests covered by USP 87 and USP 88, please see our article covering in-vitro vs. in-vivo cytotoxicity testing.
Reusable device processing and cleaning procedures are also essential considerations for cytotoxicity assessment. Reusable devices may require cytotoxicity testing for initial use and device use following recommended reprocessing.
What do elution tests assess?
Elution tests are designed for evaluating extracts from plastic materials (polymeric materials). Elution tests for cytotoxicity are beneficial for assessing high-density materials and evaluating dose-response in vitro. Elution testing methods allow for sample extraction at multiple time points and temperature conditions.
How are elution tests performed?
For elution testing, product or material extracts are prepared using a 0.9% sodium chloride injection or mammalian cell culture media. Often 0.1 g of elastomeric materials or 0.2 g of other plastic material is used per milliliter of extraction medium. Polyurethane film containing either zinc diethyldithiocarbamate (ZDEC)2 or zinc dibutyl dithiocarbamate (ZDBC) is used as a positive control.
Next, L-929 mammalian fibroblast cells are grown in a serum-supplemented minimum essential medium (MEM), and a cell suspension is prepared. Equal amounts of the L-929 cell suspension are added to culture plates with a 35-millimeter diameter to create a single-layer cell culture. After L-929 cells have been cultured to reach the appropriate confluence, the cell culture medium is aspirated from the plates and replaced with extracts from material samples, positive controls, and negative controls. Extracts from cell culture media remain without dilutions. Extracts prepared with the Sodium Chloride Injection are diluted to a 25% extract concentration with a serum-supplemented cell culture medium. All samples and controls are cultured in duplicate or triplicate at 37 ± 1°C in a humidified incubator containing 5 ± 1% of carbon dioxide.
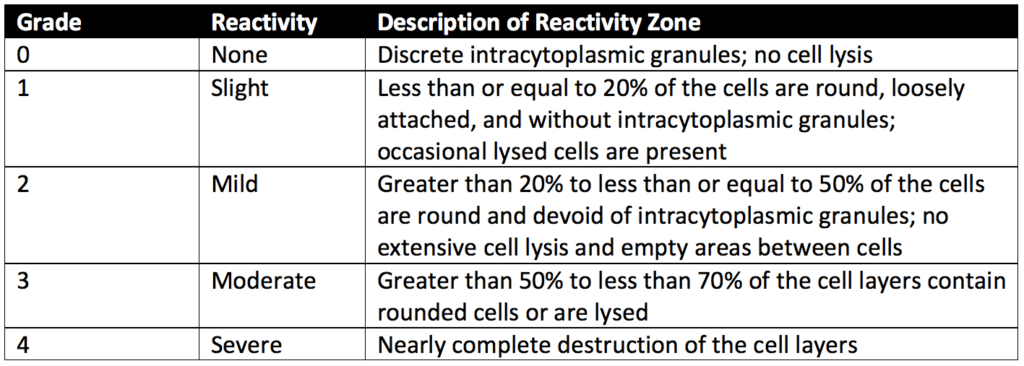
After incubation, cells exposed to samples, positive controls, and negative controls are stained or evaluated without staining under the microscope. The biological reactivity of the cells exposed to the sample or sample extracts is rated on a scale of 0-4 (see Table 1 below). The biological reactivity is determined by assessing the nonlethal injury of the cells (cellular degeneration) and any structural defects (malformations) the cells have. The agar diffusion test is valid if the observed responses to the negative controls are grade 0 and the Positive controls are all at least grade 3. The sample meets the agar diffusion test’s requirements if the samples’ biological responses are not greater than grade 2.
Summary
Overall, cytotoxicity testing evaluates the toxicity of the polymeric materials used by medical devices and products. Elution testing is one of three standard tests for cytotoxicity. Elution tests are designed for assessing the cytotoxicity of sample extracts. In these tests, L-929 fibroblast cells are cultured to reach confluence. Next, extract medium from product samples, positive controls, and negative controls are added to the cells. All samples and controls are cultured with the L-929 cells in duplicate or triplicate. Finally, the cells are then evaluated to determine the toxicity of the product samples. The types of cytotoxicity testing to perform for your medical device or product depend upon the final product, the final product’s intended use, and the materials the final product is made of and packaged within. Make sure you choose a contract testing organization that can support you with appropriate cytotoxicity testing for your unique medical device’s or product’s needs.
Ethide Labs is a contract testing organization that specializes in Cytotoxicity Testing. Ethide Labs provides in-vitro cytotoxicity tests in-house and outsources in-vivo cytotoxicity work for toxicity testing of medical devices, products, and drugs. Ethide Labs also offers Bacterial Endotoxin Testing, Sterilization Validations, Bioburden Testing, Microbiology Testing, Environmental Monitoring, EO Residual Testing & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <87> Biological Reactivity Tests, In Vitro. Rockville, MD, USA. 2021. (USPC <87>).
Share this in your social networks


