What is systemic injection cytotoxicity testing for medical devices and drugs?
What is in-vivo cytotoxicity testing and could it be important for your medical device or product?
Cytotoxicity testing is most commonly performed in-vitro. However, in-vivo tests are required in certain instances to determine the biological reactivity of mammalian cell cultures to contact with elastomeric plastics and other polymeric materials that will come in direct or indirect patient contact during medical device or product use. Systemic injection testing is one of three in-vivo testing methods for cytotoxicity. Systemic injection testing uses product extracts for testing procedures. The types of tests to be performed for your medical device or product depend upon the final product, the final product’s intended use, and the materials the final product is made of and packaged within. Processing and cleaning procedures of reusable devices are also important considerations for cytotoxicity assessment. Reusable devices may require cytotoxicity testing for both their initial use and their use following recommended reprocessing instructions.
Classification of Plastics
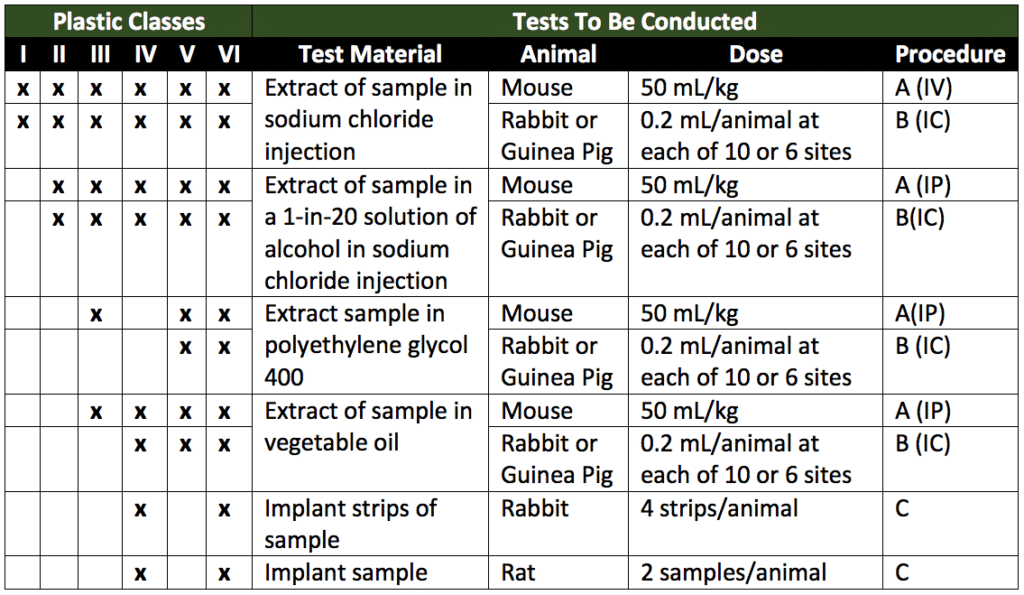
Plastic polymers evaluated for toxicity fall under six classes. Plastics are classified based on their extractants (solutions in which polymer extracts will be prepared). Plastics are also classified based on the route of administration (the end product the plastics will be used for). Lastly, plastics are classified by their material properties. These tests are directly related to the intended end-use of the plastics. The extractant(s) choice is based on any solution(s) in the preparations of the plastics which they are likely to be in contact with. Table 1 below (a reproduction of Table 1 of USP 88) summarizes the six plastic classes based on test material, animal model, dosage, and procedure (route of administration). These classifications summarize the tests needed for various plastics.
In Table 1, the tests required for each plastic class are indicated by “x” in the appropriate columns. Under the procedure column, A (IP) stands for systemic injection test (intraperitoneal), B (IC) stands for intracutaneous test (intracutaneous), and C stands for implantation test (intramuscular or subcutaneous implantation).
Introduction to systemic injection testing for cytotoxicity
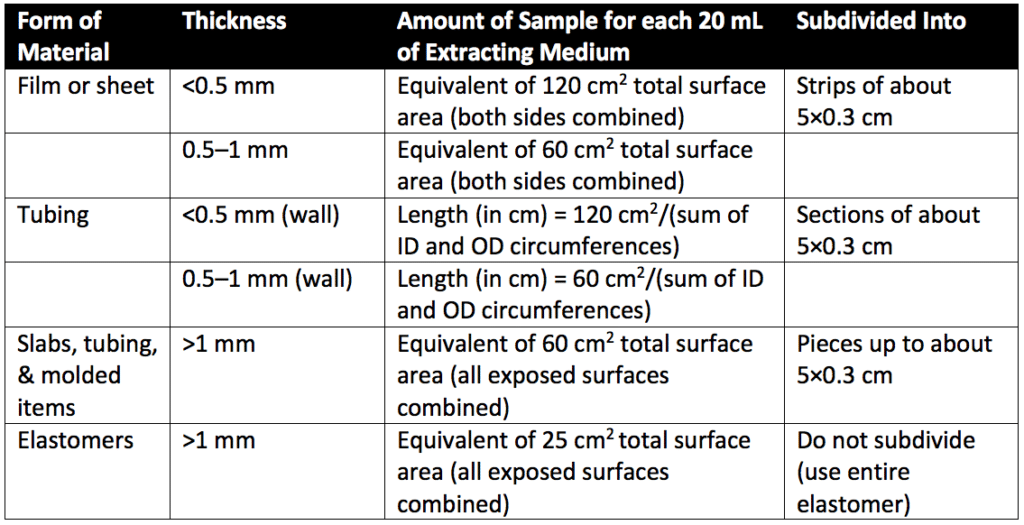
Systemic injection testing is designed to determine the systemic biological responses of animals to plastics (or other polymers) by a single-dose injection of sample extracts from medical devices or therapeutic products. Systemic injection testing and intracutaneous testing may be performed using the same extracts. Extracts are prepared depending on the heat resistance of the material being assessed. Thus, extracts are prepared at either 50°, 70°, or 121°C. Extracts are often classified by the type of plastic (Table 1) and extract temperature. For example, a class IV plastic extracted at 121° would be referred to as IV-121°. Plastics used for containers for oral or topical products do not apply for Table 1 classification. Additionally, Table 1 classifications do not apply to natural elastomers. Natural elastomers are tested in sodium chloride injection and vegetable oils only. Sample sizes for extract preparation are detailed in Table 2 below.
Extraction conditions should not cause physical changes such as fusion or melting of the sample pieces. Fusion or melting of sample pieces would decrease the available surface area and jeopardize the results.
In general, extraction conditions of most importance are:
- Contact of the extracting medium with the available surface area of the plastic
- Extraction temperature
- Extraction time
- Proper extract cooling
- Proper extract agitation
- Proper extract decanting
- Aseptic handling and storage of the extracts following extraction
Skin reaction evaluation for systemic injection testing
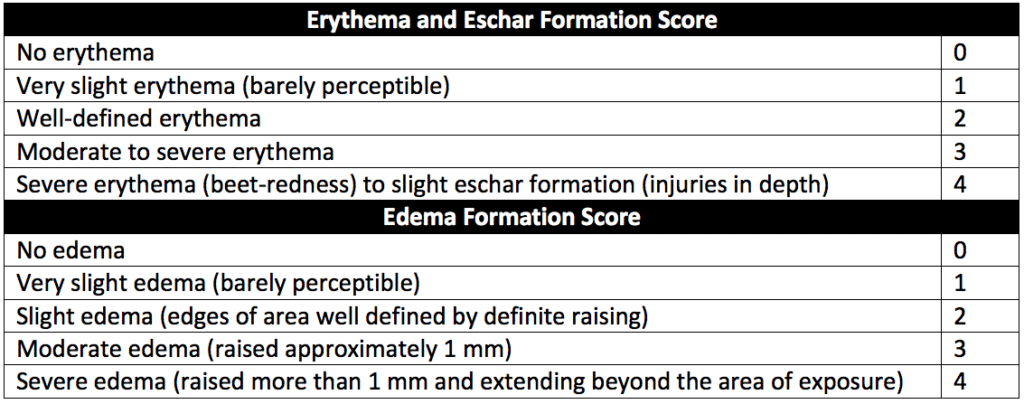
For edema scoring, the scale described in Table 2 of USP 88 (reproduced as Table 3 below) excludes noninflammatory (mechanical) edema from the blank or extraction fluid. Oil residue at the injection site should not be misinterpreted as edema. Edematous tissue blanches when gentle pressure is applied.
The scale in Table 3 below uses the scoring system described in “Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes” (Draize JH et al).
The mice are assessed immediately after injection and at 4, 24, 48, and 72 hours after injection. Suppose none of the animals treated with the sample extract show a significantly greater biological reactivity than the animals treated with the blank (at all observation times). In that case, the polymeric material under assessment meets the requirements of this test.
The sample does not meet the test requirements if one or more of the following three situations occur. Situation one is two or more mice die. Situation two is abnormal behavior, such as convulsions or prostration, occurs in two or more mice. The final situation is if a body weight loss greater than 2 grams occurs in three or more mice. Suppose any animals treated with the sample extract show only slight signs of biological reactivity, and not more than one animal shows gross symptoms of biological reactivity or dies. In that case, the test is repeated using an additional 10 mice. On the repeat test, all 10 animals treated with the sample extract must show no significant biological reactivity above the blank animals during the observation periods to pass the systemic cytotoxicity testing requirements.
Summary
Overall, cytotoxicity testing evaluates the toxicity of the polymeric materials used by medical devices and products. Systemic cytotoxicity testing determines the systemic response of an animal (mice) to the injection of extracts from polymeric materials. A systemic injection is one that is either intravenous or intraperitoneal. Following systemic injection, animals are assessed immediately following injection and at 4, 24, 48, and 72 hours after initial injection for signs of biological reactivity. If none of the animals treated with the sample extract show a significantly greater biological reactivity than the animals treated with the blank, the polymeric material under assessment meets the requirements of the systemic injection test.
Ethide Labs is a contract testing organization that specializes in Cytotoxicity Testing. Ethide Labs provides in-vitro cytotoxicity tests in-house and outsources in-vivo cytotoxicity work for toxicity testing of medical devices, products, and drugs. Ethide Labs also offers Bacterial Endotoxin Testing, Sterilization Validations, Bioburden Testing, Microbiology Testing, Environmental Monitoring, EO Residual Testing & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <88> Biological Reactivity Tests, In Vivo. Rockville, MD, USA. 2021. (USPC <88>).
Draize JH, Woodward G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. The Journal of Pharmacology and Experimental Therapeutics. 1944;82:377–390.
Share this in your social networks


