How Is Intracutaneous Cytotoxicity Testing Performed?
What is in-vivo cytotoxicity testing, and why is it needed for your medical device or product?
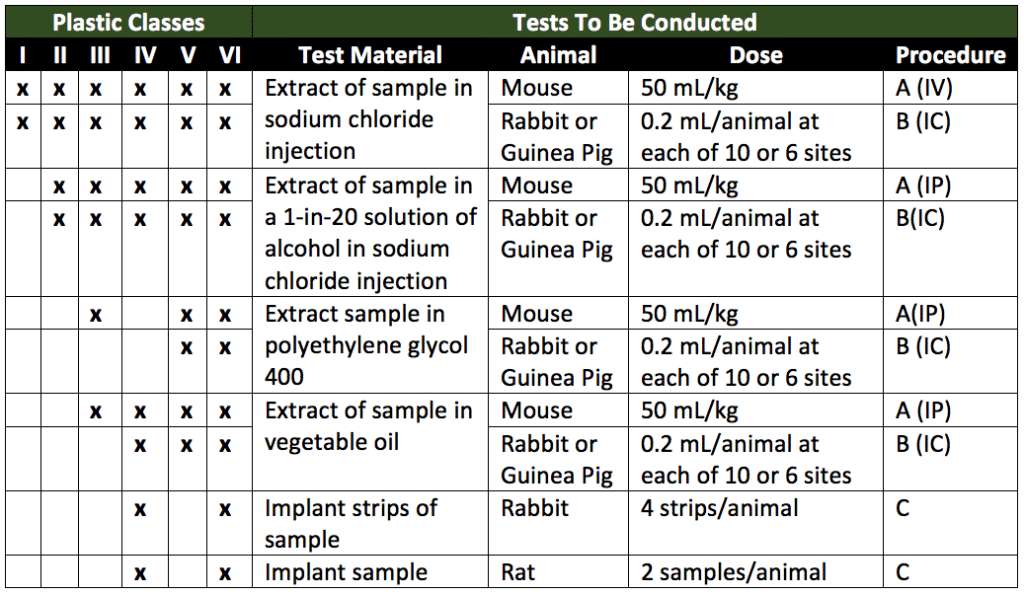
Plastic polymers evaluated for toxicity fall under six classes. Plastics are classified based firstly on their extractants (solutions in which polymer extracts will be prepared). The extractant(s) choice is based on any solutions in the preparations the plastics are likely to be in contact with. Secondly, plastics are classified by their route of administration (the end product the plastics will be used for). Lastly, plastics are ranked based on their material properties. All cytotoxicity tests are directly related to the intended end-use of the plastics under assessment. Table 1 below (a reproduction of Table 1 of USP 88) summarizes the six plastic classes based on test material, animal model, dosage, and procedure (route of administration). These classifications summarize the tests that will be needed for each category of plastic. In Table 1, the tests required for each plastic class are indicated by “x” in the appropriate columns. Under the procedure column in Table 1, A (IP) stands for a systemic injection test (intraperitoneal), B (IC) stands for an intracutaneous test (intracutaneous), and C stands for implantation test (intramuscular or subcutaneous implantation). Intracutaneous allergy testing and tests that meet USP 88 and ISO 10993 intracutaneous reactivity tests in animals will be covered.
Classification of Plastics
Plastic polymers evaluated for toxicity and intracutaneous reactivity fall under six classes. Plastics are classified based on their extractants (solutions in which polymer extracts will be prepared). Plastics are also classified based on the route of administration (the end product the plastics will be used for). Lastly, plastics are classified by their material properties. These tests are directly related to the intended end-use of the plastics. The extractant(s) choice is based on any solution(s) in the preparations of the plastics which they are likely to be in contact with. Table 1 below (a reproduction of Table 1 of USP 88) summarizes the six plastic classes based on test material, animal model, dosage, and procedure (route of administration). These classifications summarize the tests needed for various plastics.
In Table 1, the tests required for each plastic class are indicated by “x” in the appropriate columns. Under the procedure column, A (IP) stands for systemic injection test (intraperitoneal), B (IC) stands for intracutaneous test (intracutaneous), and C stands for implantation test (intramuscular or subcutaneous implantation).
Introduction to intracutaneous testing for cytotoxicity
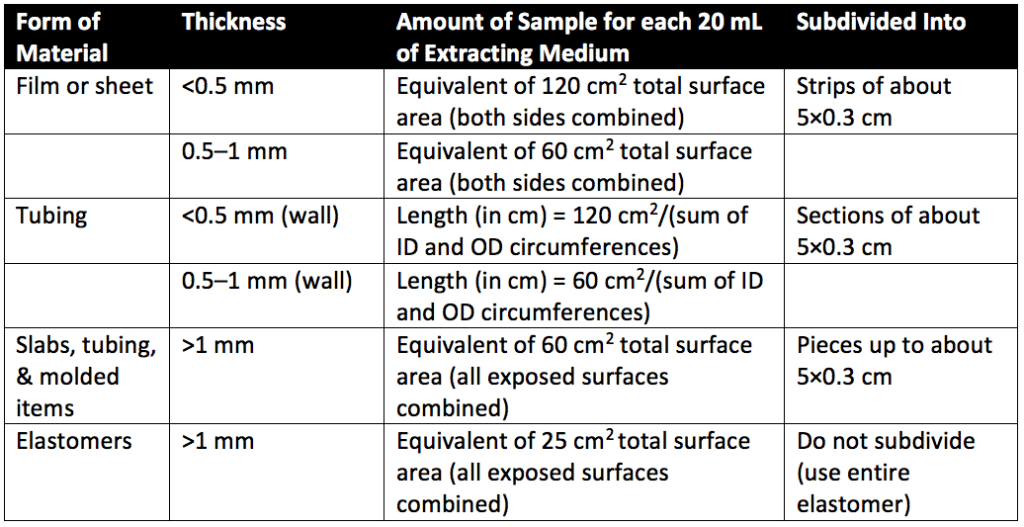
Intracutaneous allergy and reactivity testing is designed to determine the local biological responses of animals to plastics (or other polymers) by a single-dose injection of sample extracts from medical devices or therapeutic products. An intracutaneous injection is an injection between the layers of the skin. Systemic injection testing and intracutaneous testing may be performed using the same extracts. Extracts are prepared depending on the heat resistance of the material being assessed. Thus, extracts are prepared at either 50°, 70°, or 121°C. Extracts are often classified by the type of plastic (Table 1) and extract temperature. For example, a class IV plastic extracted at 121° would be referred to as IV-121°. Plastics used for containers for oral or topical products do not apply for Table 1 classification. Additionally, Table 1 classifications do not apply to natural elastomers, which are tested in sodium chloride injection and vegetable oils only. Sample sizes for extract preparation are detailed in Table 3 of USP 88 (reproduced as Table 2 below).
Extraction conditions should not cause physical changes such as fusion or melting of the sample pieces. Fusion or melting of sample pieces would result in a decrease in the available surface area and jeopardize the results.
In general, extraction conditions of most importance are:
- Contact of the extracting medium with the available surface area of the plastic
- Extraction temperature
- Extraction time
- Proper extract cooling
- Proper extract agitation
- Proper extract decanting
- Aseptic handling and storage of the extracts following extraction
Skin reaction evaluation for intracutaneous cytotoxicity testing
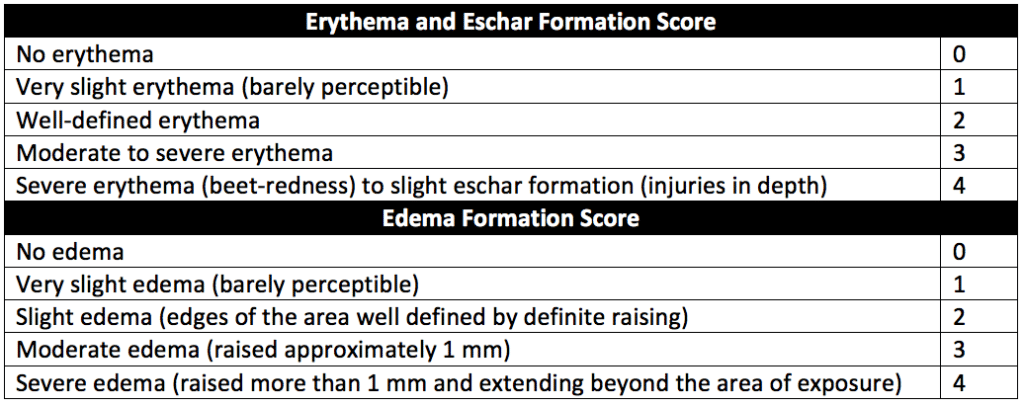
For intracutaneous reactivity and allergy testing, the edema scoring scale described in Table 2 of USP 88 (reproduced as Table 3 below) excludes noninflammatory (mechanical) edema from the blank or extraction fluid. Oil residue at the injection site should not be misinterpreted as edema. Edematous tissue blanches when gentle pressure is applied.
The scale in Table 3 below uses the intracutanous reactivityscoring system described in “Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes” (Draize JH).
How are intracutaneous cytotoxicity tests performed?
Intracutaneous allergy and reactivity testing determines the local response of an animal (rabbits or guinea pigs) to intracutaneous injection of material extracts. For testing, animals are shaved, and any loose hair is vacuumed away. Care is taken to prevent any mechanical irritation or trauma of the animal’s skin before injection. Each extract is shaken vigorously before injection to ensure an even distribution of the extracted matter. More than one extract from a given material can be used per rabbit or guinea pig (if the test results will not be affected). For each sample, two animals are injected intracutaneously. One side of the animal is injected with the sample extract, and the other side is injected with the negative control (Blank). Table 5 of USP 88 describes this process and is reproduced in Table 4 below. Note that blanks are composed of the media used for extract preparation. Media used for blanks has not come in contact with the sample materials being assessed.

After injection, injection sites are assessed for evidence of any tissue reaction such as erythema, edema, and necrosis (see Table 3 above for scoring system). All animals are observed at 24, 48, and 72 hours after injection for tissue reaction. The average erythema and edema scores for the extract and blank injection sites are determined at every scoring interval (24, 48, and 72 hours) for each rabbit or guinea pig.
After the 72-hour scoring, all erythema scores plus edema scores are totaled separately for each extract sample and blank. Each total is divided by twelve (two animals × three scoring periods × two scoring categories) to determine the overall mean score for each sample extract versus each corresponding blank. Suppose the average reaction to the sample extract is questionably greater than the average reaction to the blank (at any observation period). In that case, the test is repeated using three additional rabbits or guinea pigs. The test requirements are met if the difference between the mean score of the extract samples and the mean score of the blanks (negative controls) is 1.0 or less.
Summary
Overall, in-vivo cytotoxicity testing evaluates the toxicity of the polymeric materials used by medical devices and products. Intracutaneous cytotoxicity testing determines the local response of an animal (rabbits or guinea pigs) to the intracutaneous injection of extracts from polymeric materials. An intracutaneous injection is an injection between the layers of the skin. Following injection, animals are assessed at 24, 48, and 72 hours after initial injection for signs of erythema and edema. If the difference between the mean score of the extract samples and the mean score of the blanks (negative controls) is 1.0 or less, the material passes the intracutaneous injection test.
Ethide Labs is a contract testing organization that specializes in Cytotoxicity Testing. Ethide Labs provides in-vitro cytotoxicity tests in-house and outsources in-vivo cytotoxicity work for toxicity testing of medical devices, products, and drugs. Ethide Labs also offers Bacterial Endotoxin Testing, Sterilization Validations, Bioburden Testing, Microbiology Testing, Environmental Monitoring, EO Residual Testing & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <88> Biological Reactivity Tests, In Vivo. Rockville, MD, USA. 2021. (USPC <88>).
Draize JH, Woodward G, Calvery HO. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. The Journal of Pharmacology and Experimental Therapeutics. 1944;82:377–390.
Share this in your social networks


