What is implantation cytotoxicity testing for medical devices and drugs?
What is cytotoxicity testing, and why is it important for your medical device or product?
Cytotoxicity testing is most commonly performed in-vitro. However, in-vivo tests are required in certain instances to determine the biological reactivity of mammalian cell cultures to contact with elastomeric plastics and other polymeric materials. In-vitro testing is important to any material that will come in direct or indirect patient contact during medical device or product use. The implantation testing is one of three in-vivo testing methods for cytotoxicity. Unlike systemic injection and intracutaneous testing, implantation testing does not use product extracts for testing procedures.
The types of tests performed for your medical device or product depend upon the final product, the final product’s intended use, and the materials the final product is made of and packaged within. Processing and cleaning procedures of reusable devices are also important considerations for cytotoxicity assessment. Reusable devices may require cytotoxicity testing for both their initial use and their use following recommended reprocessing procedures.
Introduction to implantation testing for cytotoxicity
The implantation test is designed to evaluate the reaction of living tissue to the plastic and other polymers by implanting the device or therapeutic product itself into animal tissue. Material samples must be aseptically prepared and surgically placed under aseptic conditions to ensure accurate results. Implantation tests are designed for application to plastics and other polymers in the state in which they are used. In other words, if the plastic being assessed is to be exposed to any cleansing or sterilization process before its end-use, then the tests are to be conducted on a polymer sample that has undergone the recommended cleaning or sterilization processes. The factors below should be considered and tested when evaluating polymers for their intended use.
Factors that can impact the suitability of a material for its intended use are:
- Material composition
- Material processing and cleaning procedures
- Contacting media(s)
- Inks
- Adhesives
- Absorption properties
- Adsorption and permeability of preservatives
- Conditions of storage
Skin reaction evaluation for implantation cytotoxicity testing
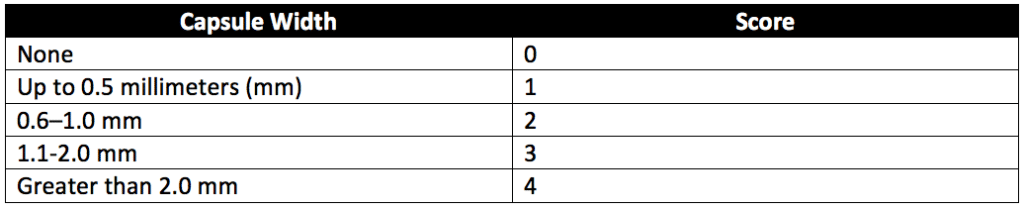
For implantation testing scoring, the scale described in Table 6 of USP 88 (reproduced as Table 1 below) defines how implants are assessed following surgical encapsulation in test animals.
How are implantation cytotoxicity tests performed?
The implantation tests are performed by surgically introducing a sample of the material under evaluation into a test animal. When surgically implanting sample pieces, proper aseptic preparation of the implant strips and adequate placement of the strips under aseptic conditions is vital to implantation testing success. There are two forms of implantation testing, intramuscular implantation in rabbits and subcutaneous implantation in rats. Intramuscular implantation testing uses healthy adult New Zealand rabbits. For intramuscular implantation, test specimens are placed into needles for implantation delivery. Materials that have physical characteristics that are unsuitable for needle delivery may use the subcutaneous rat implantation model.
Intramuscular implantation in rabbits
For intramuscular implantation, eight strips of the sample material and four USP High-Density Polyethylene RS strips are prepared. Each strip should measure not less than 10 × 1 mm. To avoid hurting tissues upon implantation, the edges of the strips should be as smooth as possible. Each strip is implanted using a hypodermic needle (15–19 gauge) with an intravenous point and a sterile trocar. Strips are embedded by inserting the sanitary plastic strips into the hypodermic needle and then using the needle to implant the strips into the rabbits’ muscular tissues.
Healthy adult rabbits weighing not less than 2.5 kilograms and with paravertebral muscles large enough for test strip implantation are used. Only paravertebral muscles are used for implantation sites. After the animals are anesthetized, the fur along the implantation areas of each animal is clipped. Then the implantation sites are cleaned. Next, four sample strips are implanted into the paravertebral muscle on one side of each of the two rabbits’ spines. Two strips of USP High-Density Polyethylene RS are embedded in the opposite paravertebral muscle of each animal as negative controls. Animals are exposed to the implanted strips for at least 120 hours before tissue assessment. The area of the tissue surrounding the center of each implant strip is examined microscopically. Sample and control implant sites are assessed for hemorrhage, necrosis, discolorations, and infections. All observations are recorded, and encapsulation sites measured to the nearest 0.1 mm. The scoring system in Table 1 above is used for tissue assessment. After all tissue scores are taken, differences between the average scores for the sample and control sites are calculated. Samples pass the implantation test if the calculated difference does not exceed 1.0 or if the difference between the sample and control mean scores for more than one of the four implant sites does not exceed 1 for any implanted animal.
Subcutaneous implantation in rats
For subcutaneous implantation in rats, ten samples and ten controls (USP High-Density Polyethylene RS) are prepared for the experiment. To avoid hurting tissues upon implantation, the edges of the sample and control strips should be as smooth as possible. Five healthy albino rats weighing 225–350 grams are used for testing. Implantation sites along the rat’s spinal columns are prepared similarly to the implantation sites for rabbits. The rats are anesthetized via AAALAC guidelines. After the initial incision, blunt dissection is used to separate the fascia connecting skin to muscle to form a pocket underneath the skin lateral to each side of the incision. A sterile sample is inserted into each tissue pocket, and the incision closed with wound clips or sutures. Two test samples and two control samples are implanted in each of the five rats. The animals are kept for at least seven days following sample implantation before tissues are assessed.
Similar to implantation in rabbits, the area of the tissue surrounding the center of each implant strip is examined microscopically. Sample and control implant sites are assessed for hemorrhage, necrosis, discolorations, and infections. All tissue observations are recorded, and encapsulation sites measured to the nearest 0.1 mm. The scoring system in Table 1 above is used for tissue assessment. After all tissue scores are taken, differences between the average scores for the sample and control sites are calculated. The implantation test is passed if the difference does not exceed 1.0 or if the difference between the sample and control mean scores for more than one of the four implant sites does not exceed 1 for any implanted animal.
Summary
Overall, in-vivo cytotoxicity testing evaluates the toxicity of the polymeric materials used by medical devices and products. Implantation cytotoxicity testing assesses the reaction of living tissue to direct contact with plastic materials and other polymeric materials used in medical devices or therapeutic products. For implantation, material samples are introduced to the animals either through intramuscular injection or subcutaneous implantation. Following implantation, animal implantation sites are assessed after at least 120 hours (2 days) for rabbits and after seven days for rats. Samples with differences between the mean score of the samples and the mean score of the USP High-Density Polyethylene RS (negative controls) that are 1.0 or less pass the implantation test.
Ethide Labs is a contract testing organization that specializes in Cytotoxicity Testing. Ethide Labs provides in-vitro cytotoxicity tests in-house and outsources in-vivo cytotoxicity work for toxicity testing of medical devices, products, and drugs. Ethide Labs also offers Bacterial Endotoxin Testing, Sterilization Validations, Bioburden Testing, Microbiology Testing, Environmental Monitoring, EO Residual Testing & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <88> Biological Reactivity Tests, In Vivo. Rockville, MD, USA. 2021. (USPC <88>).
Share this in your social networks


