Which Is The Best Sterilization Method For Your Medical Device?

What is sterilization?
Sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization is related to the term sterile, which means a complete absence of viable microorganisms that have the potential to reproduce. Sterilization processes kill any microorganisms inside products that were obtained during manufacturing. Often products that undergo sterilization are chemically or thermally sterilized after being placed in their final packaging containers. In this article we will cover available sterilization methods, the type of sterilization method you should select for your device’s materials based on our sterilization method comparison chart, dry heat sterilization methods, moist heat sterilization methods, ethylene oxide sterilization methods, and much more!
What are my options for medical device sterilization?
There are seven primary techniques for medical device sterilization. These methods are steam sterilization, radiation sterilization, dry heat sterilization, sterilization by filtration, gas sterilization (such as ethylene oxide sterilization), vapor sterilization, and liquid sterilization. Of these sterilization methods, gas sterilization, vapor sterilization, and liquid sterilization techniques are chemical sterilization processes. As you might expect, steam and dry heat sterilization are thermal sterilization processes. Table 1 below contains brief descriptions of each sterilization technique.
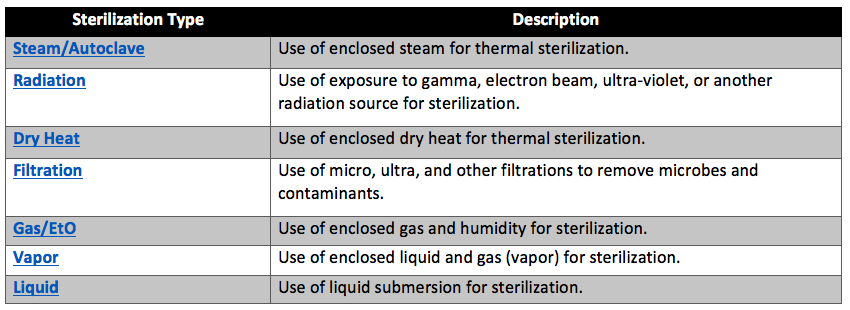
Which sterilization method is best for your medical device?
Deciding which sterilization technique is best for your medical device will primarily depend on the materials your medical device is made of. Nearly all devices that contain biologics will require sterilization by filtration for the biological component of the device. The most inexpensive and popular method of sterilization is steam sterilization. Devices made with metals, or other materials compatible with steam sterilization will be sterilized using autoclaves. The second most common method of sterilization is ethylene oxide gas sterilization. Ethylene oxide gas sterilization is often used for heat-sensitive materials. However, some plastics heavily absorb ethylene oxide (EtO) and contain too many toxic EtO residuals for certain medical device applications. Radiation is the most effective “cold” type of sterilization method. However, radiation sterilization is more expensive, harder to outsource, and requires the use of materials that will not break down upon radiative exposure. Vapor phase sterilization is sometimes used instead of steam for faster results. Liquid sterilization is very rare and often never used for medical devices.
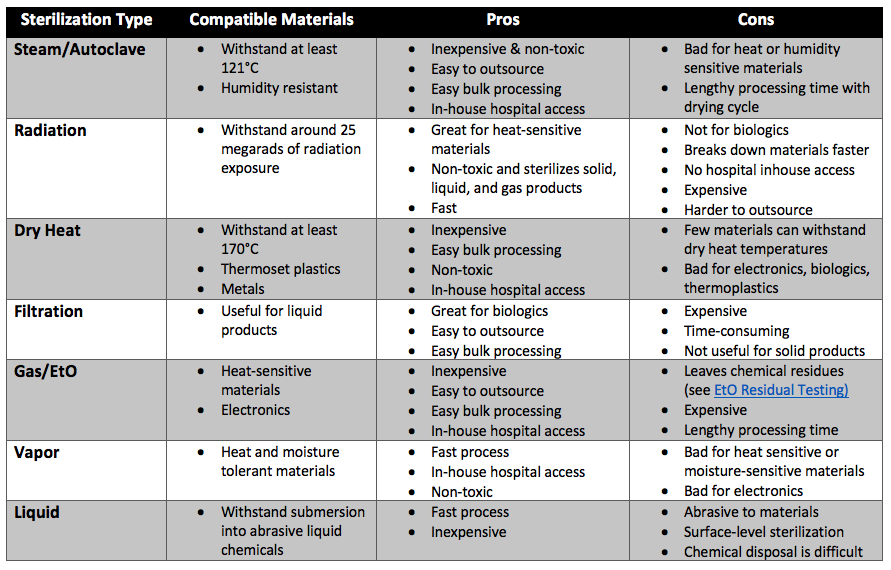
Summary
Overall, sterilization is any process that removes, kills, or deactivates all forms of life. There are seven primary techniques for medical device sterilization. These methods are moist heat sterilization, radiation sterilization, dry heat sterilization, sterilization by filtration, gas sterilization (such as ethylene oxide sterilization), vapor sterilization, and liquid sterilization. Deciding which sterilization technique is best for your medical device will primarily depend on the materials your medical device is made of. Consult the comparison chart in this article to know which type of sterilization method is best for your medical device or product. Further, if outsourcing regulatory tests, ensure you choose a contract testing organization that can support you with appropriate sterilization validations for your unique medical device needs.
Ethide Labs is a contract testing organization that specializes in Sterilization Validations and Sterility Testing. Ethide Labs also offers Microbiology Testing, Bioburden Testing, Bacterial Endotoxin Testing, Ethylene Oxide Residual Testing, Cytotoxicity Testing, Environmental Monitoring & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
International Organization for Standardization. Sterilization of health care products- Moist heat- Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices. Geneva (Switzerland): ISO; 2006. (ISO 17665-1:2006/(R)2016).
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <1115> Bioburden Control of Non-Sterile Drug Substances and Products. Rockville, MD, USA. 2021. (USPC <1115>).
United States Pharmacopeial Convention. <1116> Microbiological Control & Monitoring of Aseptic Processing Environments. Rockville, MD, USA. 2021. (USPC <1116>).
United States Pharmacopeial Convention. <1211> Sterility Assurance. Rockville, MD, USA. 2021. (USPC <1211>).
United States Pharmacopeial Convention. <1229.6> Liquid-Phase Sterilization. Rockville, MD, USA. 2021. (USPC <1229.6>).
United States Pharmacopeial Convention. <1229.11> Vapor Phase Sterilization. Rockville, MD, USA. 2021. (USPC <1229.11>).
Share this in your social networks


