Sterilization of Bacteria, Fungi And Virus For Medical Devices
What is sterilization?
Sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization is related to the term sterile, which means a complete absence of viable microorganisms or microbes that have the potential to reproduce. Thus, sterile products that undergo sterilization are often chemically or heat sterilized after being placed in their final packaging. The chemical or heat sterilization kills any microorganisms inside the products (obtained during manufacturing and packaging). This chemical or heat sterilization process after final product packaging is known as terminal sterilization.
Four main methods sterilize items:
- Heat
- Gas
- Radiation
- Filtration
Another way to classify these four sterilization methods is:
- Thermal
- Moist
- Dry
- Nonthermal
- Filtration
- Radiation
- Chemical
- Gaseous
- Liquid
Which microorganisms must sterilization processes eliminate?
Microbial data for growth, survival, and death kinetics comes from work performed in laboratory conditions. However, microorganisms found in manufacturing environments are commonly under nutritional, chemical, dehydration, or other stress that laboratory conditions do not simulate. Therefore, known microbial data may not be as predictive of manufacturing environments’ microbial growth, survival, and death kinetics. Three primary microbe types require appropriate sterilization (elimination) before a medical device is used: bacteria, fungi (yeast and mold), and viruses.
Bacteria
Bacteria come in two flavors, gram-positive or gram-negative. The distinction between gram-positive or negative bacteria is the presence of a cellular envelope. Gram-positive bacteria do not contain an outer cell wall, while gram-negative bacteria do. The outer cell wall of gram-negative bacteria contains lipopolysaccharide (LPS), a harmful endotoxin.
Examples of common gram-negative bacteria are Pseudomonas, Escherichia coli, and Salmonella. Examples of common gram-positive bacteria are Staphylococcus, Streptococcus, Bacillus, and Clostridium.
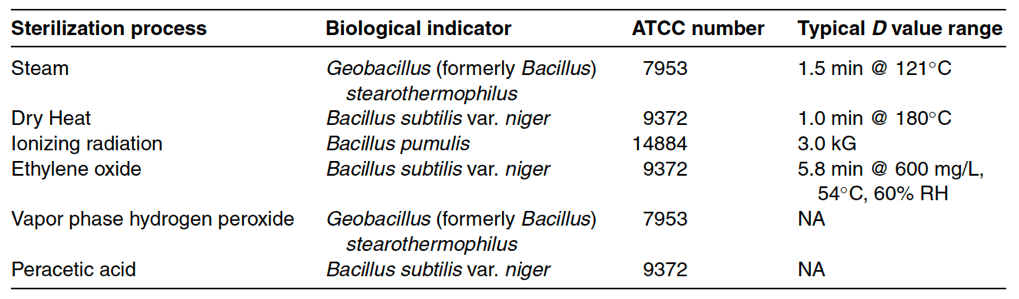
Bacteria are also classified as aerobic, anaerobic, or facultative. Aerobic bacteria require oxygen to grow, while anaerobic bacteria thrive in environments without oxygen. Facultative bacteria can grow in both aerobic and anaerobic environments. Some bacteria have spore forms. Bacterial spore formation occurs when a stressed bacterial cell develops an outer shell to protects itself from adverse environmental conditions such as heat, chemicals, and nutrient depletion. The most common spore formers are Bacillus and Clostridium species. As bacterial spore forms are hundreds of times more difficult to destroy than traditional bacteria, Bacillus and Clostridium species are used as biological indicators for sterilization processes (see Table 17-1). As biological indicators, Bacillus and Clostridium measure the effectiveness of various sterilization methods.
Fungi
Interestingly, the cellular structures of fungi are similar to human cells. Fungi, like human cells, are eukaryotic cells. Unlike prokaryotic bacterial cells, fungal cells are challenging to destroy. Care must be taken to ensure that the methods used to kill fungal cells will not residually kill human cells. Only about 10% of fungi are human pathogens. Candida species and some dermatophytes are the only fungi known to transmit from person to person. Out of the two species, Candida is the most common fungal infection.
Virus
Unlike bacteria and fungi, viruses do not need nutrients to survive. Viruses do not need food because they are tiny intracellular parasites that use host cells to multiply and survive. Viruses are inactivated by heat at relatively low temperatures (65◦C or above). Viruses are also easily inactivated using surface disinfectants and do not survive well in manufacturing environments. For this reason, the risk of viral contamination in manufacturing environments is extremely low.
What are microbial growth and death kinetics?
Microbial growth (whether bacterial, fungal, or viral) is exponential. Thus, when low-level contamination of a product occurs, it cannot be identified immediately. There is a lag time before the contaminating microbes have grown to the point where they can be measured and the contamination verified. This lag phase varies between minutes to years depending upon the microbial species, product storage temperature, available nutrients, and other factors that impact microbial growth kinetics. For sterilization, relevant lag times are on the scale of minutes to hours. Once microbial growth starts, it progresses rapidly. For example, a typical bacterial cell can produce over 1,300,000 cells in 8 hours! Eventually, microbial growth plateaus to a stationary phase when critical nutrients are depleted, oxygen diffusion is inhibited, or toxic metabolites accumulate. The approximate population where stationary phases exist is 10,000,000 cells/milliliter.
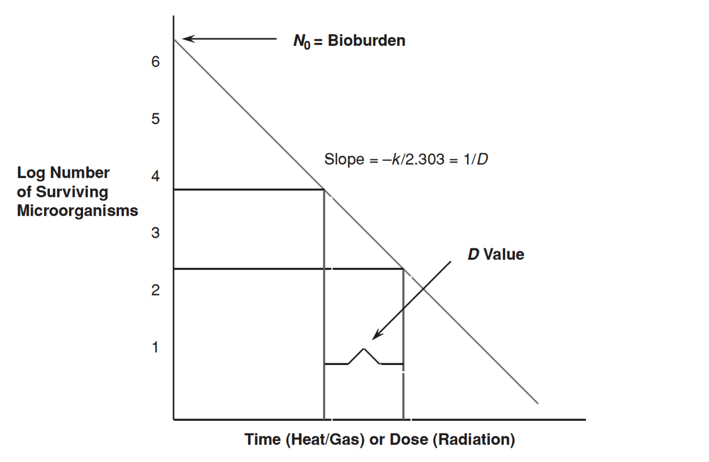
Microbial death kinetics, like growth kinetics, are exponential. Figure 17-2 below is an example of a microbial death kinetic plot. Microbial death plots can be used to calculate values and constants that describe microbial death kinetics for organisms. The most common values and constants used in microbial death kinetic studies and sterilization validations are bioburden, D value, Z value, and F value. Bioburden is described below. D values, Z values, and F values are not discussed in this article.
What is bioburden?
The “bio” in bioburden refers to live biological organisms, and the “burden” in bioburden refers to the concentration of the viable biological organisms. Thus, bioburden is the concentration or quantity of microorganisms in a given area or from a particular sample. In other words, bioburden is the initial population of a microorganism before a sample or item is sterilized. The higher the concentration of viable organisms on a device or product, the higher the burden is to kill those organisms, whether it is killing the organisms through sterilization procedures or killing the organisms through the effort of the human immune system. United States Pharmacopeia (USP) Chapter 1111 requires a bioburden limit of not more than one thousand colony forming units per gram (or milliliter) guidelines for raw materials, excipients, and bulk drug substances.
Summary
Overall, sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization for medical devices and products is critical for ensuring patient safety during product use. Three primary microbe types require appropriate sterilization (elimination) before medical devices are used: bacteria, fungi (yeast and mold), and viruses. Microbial growth (whether bacterial, fungal, or viral) is exponential. Thus, when low-level contamination of a product occurs, it cannot be identified immediately. There is a lag time before the contaminating microbes have grown to the point where they can be measured and the contamination verified. Microbial death kinetics, like growth kinetics, are exponential. Microbial death plots can be used to calculate values and constants that describe microbial death kinetics for organisms. The most common bacterial spore formers, Bacillus and Clostridium species, are used as biological indicators. As biological indicators, Bacillus and Clostridium measure the effectiveness of various sterilization methods. All in all, ensure you choose a contract testing organization that can provide appropriate sterilization validations for your product needs.
Ethide Labs is a contract testing organization specializing in Sterilization Validations & Sterility Testing. Ethide Labs also offers EO Residual Testing, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, Bioburden Testing, Package Integrity Testing & Environmental Monitoring services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
International Organization for Standardization. Sterilization of health care products- Moist heat- Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices. Geneva (Switzerland): ISO; 2006. (ISO 17665-1:2006/(R)2016).
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <1111> Microbiological Examination Of Nonsterile Products: Acceptance Criteria For Pharmaceutical Preparations And Substances For Pharmaceutical Use. Rockville, MD, USA. 2021. (USPC <1111>).
United States Pharmacopeial Convention. <1115> Bioburden Control of Non-Sterile Drug Substances and Products. Rockville, MD, USA. 2021. (USPC <1115>).
United States Pharmacopeial Convention. <1116> Microbiological Control & Monitoring of Aseptic Processing Environments. Rockville, MD, USA. 2021. (USPC <1116>).
United States Pharmacopeial Convention. <1211> Sterility Assurance. Rockville, MD, USA. 2021. (USPC <1211>).
Share this in your social networks


