Liquid vs. Radiation Sterilization For Medical Devices
What is sterilization, and why is it essential for sterile products?
Sterilization keeps patients safe from toxins and microbial illnesses when therapies or devices are consumed or used. Sterilization is any process that removes, kills, or deactivates all forms of life. Under the strictest definition of sterility, an item or product is sterile when there is the complete absence of viable microorganisms (bacteria, yeasts, viruses, and molds). For regulatory purposes, sterility is defined by acceptance criteria based on calculated contamination probability. An acceptable level of contamination risk for most items is the probability of a single contaminated product out of a million manufactured products. However, sterility criteria may be more stringent or lax depending upon the intended use of the medical device or product. Commonly, sterile products undergo sterilization processes that utilize chemicals, heat, radiation, or filters. Sterilization kills any microorganisms products collect during manufacturing. A less common version of sterilization is liquid sterilization. In contrast, radiation sterilization for healthcare products is trending for medical device sterilization services.
What is radiation sterilization For healthcare Products?
Sterilization by radiation is a non-thermal sterilization method that functions by destroying any microorganisms in a product with gamma radiation, beta particles (electron beam), x-ray, or ultraviolet (UV) light. Other than sterile filtration, radiation sterilization for healthcare products is the only other sterilization method that doesn’t rely on elevated temperature or chemicals for sterilization. Radiation sterilization is an excellent medical device sterilization service for products that cannot be sterilized with heat or chemicals.
What is liquid phase sterilization?
Liquid phase sterilization kills microbes through immersing products, packages, or other items in a chemical solution. Liquid sterilant lethality depends upon the chemical agent’s concentration and temperature. Examples of chemical agents (by chemical families) used as liquid sterilants are aldehydes, acids, bases, and strong oxidants. These chemicals are able to destroy resistant bacteria (spores) as well as fungi.
What products or medical devices can undergo liquid vs. radiation sterilization?
Items sterilized with radiation are the same as items that gaseous methods can sterilize. Standard devices and materials sterilized with radiation are plastics, heat-labile materials, glass, and powders. Radiation damages the nucleoproteins of microorganisms. Thus, radiation sterilization for healthcare products is not recommended for biologics. In contrast to radiation, liquid phase sterilization is useful only for sterilizing the outer surface of products and other items. Products that require the sterilization of inner surfaces or are sensitive to corrosion by liquid chemical agents should not be sterilized with liquid sterilants.
How is sterilization by radiation performed?
As a medical device sterilization service, sterilization by radiation is performed by exposing a product to gamma radiation, beta particles, or ultraviolet light. Microwave radiation is sometimes used for empty glass containers but is not considered a primary radiation sterilization method. Electromagnetic gamma radiation is the most effective radiation sterilization method due to its deep penetration. Often cobalt 60 high-energy photons are used for gamma radiation. Beta particles, as ionizing radiations, are not electromagnetic and less penetrative. Mechanically accelerated Strontium 90 creates exceptionally high energy levels and the beta particles (electron beams) needed for beta particle radiation. Ultraviolet light can also be used but only as a surface sterilizing method. The energy level of ultraviolet light is too weak to penetrate materials. Gamma sterilization is the most robust option for radiation sterilization, but beta particle (e-beam) radiation is also used for product terminal sterilization.
Radiation effectiveness is dependent on the radiation dosage and time exposure. A 12-D sterilization overkill approach is used in radiation sterilization. The 12-D stands for providing a radiation dose sufficient to produce a 12-log reduction in the D value of the most resistant microbial spore. Note that D value determination for radiation uses dosage rather than time. Typical D values for the most resistant bacterial spore (Bacillus pumulis) to radiation is 1.7 to 2.0 megarad (mRad). Commonly a radiation dosage will be 25 mRad, greater than 12-fold the D value of B. pumulis. During radiation sterilization treatment, dosimeters are placed at strategic locations to monitor radiation doses products receive throughout the sterilization process. When a product goes through a radiation sterilization conveyor (see Figure 1 below), the total radiation dosage is unequally distributed as items under sterilization are conveyed from start to finish. Total radiation percentages are greatest in conveyor areas closest to the gamma radiation source. The total radiation dose for sterilization experienced by a product may be 25 mRad. The total radiation dosage is distributed throughout the conveyor system to avoid applying an overwhelming or damaging amount of radiation at any given time during sterilization.
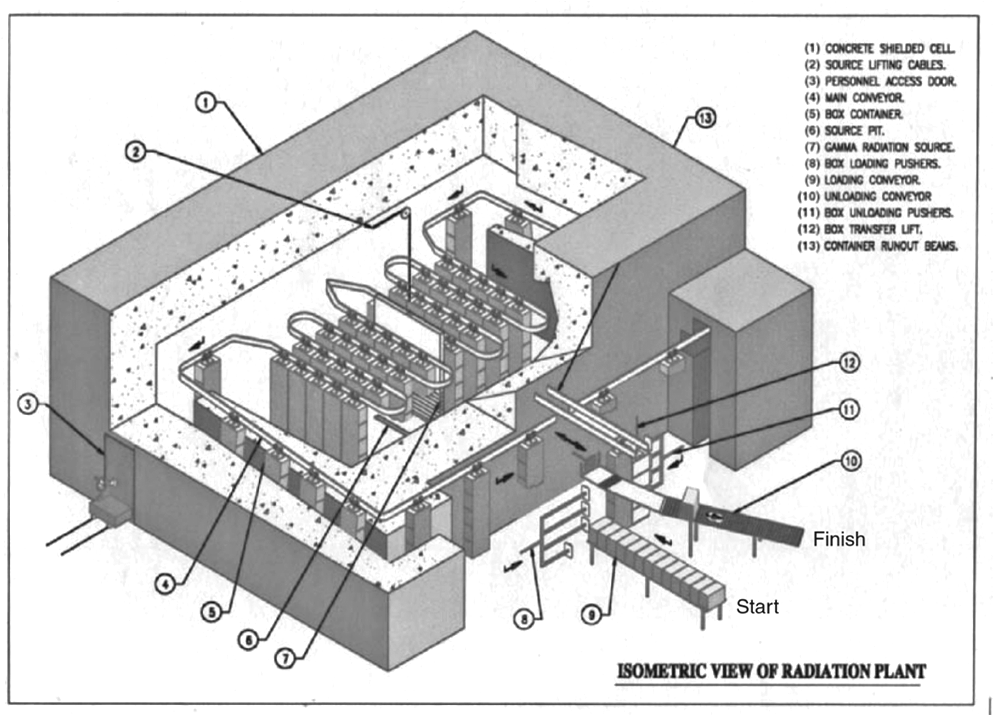
Factors that affect radiation sterilization:
- D value of the biological indicator or bioburden level of the item undergoing sterilization
- Radiation strength
- Radiation dose rate
- Conveyor speed
How is liquid phase sterilization performed?
As a medical device sterilization service, liquid phase sterilizations are simple. Objects to be sterilized are fully immersed in a sterilant solution under a designative temperature and for a set time. Then aseptic processes are used to inactivate or remove the liquid sterilant and extract sterilized products. Recontamination can occur during the liquid sterilant inactivation/removal process, unlike other sterilization methods. Thus, including steps to avoid recontamination and remove the liquid sterilant is a part of the sterilization process for liquid phase sterilization. There aren’t any widely accepted biological indicators for liquid sterilization. Thus, resistant bacterial spores like Bacillus atrophaeus or B. subtilis are used to verify sterility.
The antimicrobial activity of the sterilant is impacted by pH, concentration, processing temperature, contact time, the extent of liquid agitation (mixing) during sterilization, and the presence of particulate or cellular debris on items undergoing sterilization. Moreover, sterilants vary in their stability and interaction with product materials undergoing sterilization. When selecting a sterilant, the impact of the chemical agent on equipment, product, and packaging materials is the most critical consideration. Another consideration is personnel safety during sterilant use as liquid sterilization agents are often highly toxic and require additional safety precautions to be taken.

What are the advantages, disadvantages, and differences between liquid and radiation sterilization?
Radiation and liquid phase sterilization are alternatives to traditional heat sterilization processes. Neither sterilization method will work to sterilize products containing a biological agent. Liquid phase sterilization is a fast sterilization process. However, liquid sterilants are harsh and can only sterilize a subset of materials without causing chemical degradation or corrosion. Further, liquid phase sterilization is a surface sterilization technique. If inner surfaces need to be sterilized, this method is not a preferred sterilization technique.
The depth of radiation sterilization depends upon the type of radiation used. Gamma radiation has the deepest penetration into inner surfaces. However, there is limited understanding of the molecular transformations in drug molecules and excipients under high-energy gamma radiation exposure. An additional concern when sterilizing finished products or active pharmaceutical ingredients (APIs) with radiation is the risk of radiolytic byproduct formation (e.g., *OH) that could cause damage to the raw material, API, or product packaging system.
Radiation sterilization equipment is not commonplace in hospitals and thus is not recommended to sterilize reusable products. Advantageously, equipment and processes for liquid sterilization would be easier to implement in a hospital setting for reusable products.
Summary
Overall, medical devices, products, and therapies must be sterile. Sterilization is any process that removes, kills, or deactivates microbes. Liquid phase sterilization kills microbes through immersing products, packages, or other items in a chemical solution, whereas radiation sterilization processes are cold sterilization methods that kill microbes through exposure to gamma, beta particle (e-beam), or ultraviolet radiation. Liquid phase sterilization is useful only for sterilizing the outer surface of products and other items. Products that require the sterilization of inner surfaces or are sensitive to corrosion by liquid chemical agents should not be sterilized with this method. Standard devices and materials sterilized with radiation are plastics, heat-labile materials, glass, and powders. All in all, ensure you choose a contract testing organization that can provide appropriate sterility testing for your product needs.
Ethide Labs is a contract testing organization specializing in Sterilization Validations and Sterility Testing. Ethide Labs also offers Microbiology Testing, Bioburden Testing, EO Residual Testing, Bacterial Endotoxin Testing, Cytotoxicity Testing, Environmental Monitoring & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
International Organization for Standardization. Sterilization of health care products- Moist heat- Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices. Geneva (Switzerland): ISO; 2006. (ISO 17665-1:2006/(R)2016).
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <1211> Sterility Assurance. Rockville, MD, USA. 2021. (USPC <1211>).
United States Pharmacopeial Convention. <1229> Sterilization of Compendial Articles. Rockville, MD, USA. 2021. (USPC <1229>).
United States Pharmacopeial Convention. <1229.10> Radiation Sterilization. Rockville, MD, USA. 2021. (USPC <1229.10>).
United States Pharmacopeial Convention. <1229.6> Liquid-Phase Sterilization. Rockville, MD, USA. 2021. (USPC <1229.6>).
Share this in your social networks


