Our Services
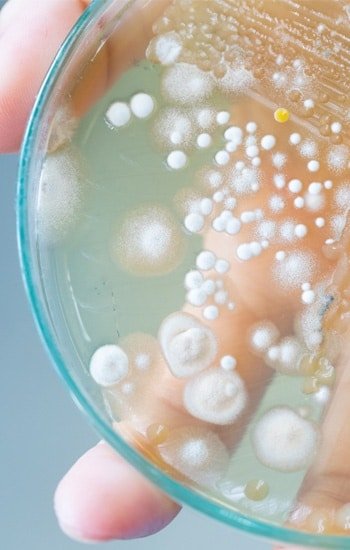
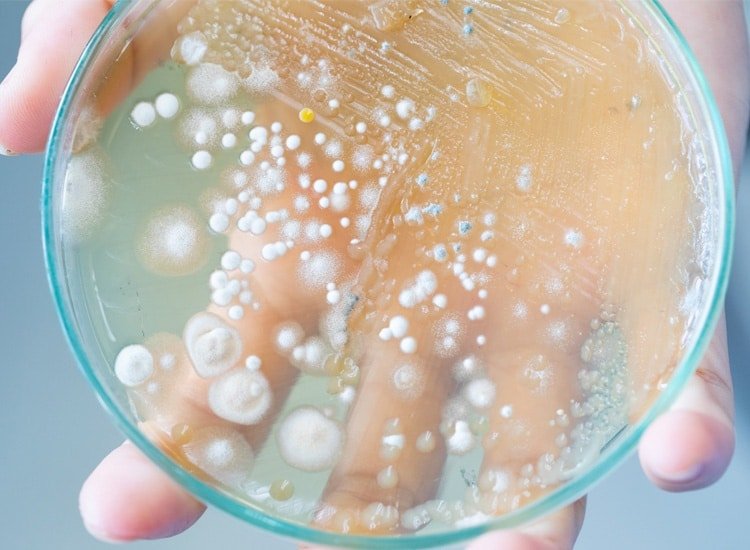
Ethide Labs is a contract testing organization specializing in EO residual and microbiology testing. We also offer bioburden testing, sterility testing, package integrity testing & environmental monitoring services for medical device companies.
Population of viable microorganisms on or in product and/or sterile barrier system.
Validation or requalification to determine the presence or absence of microorganisms on product.
Population and identification of microorganisms present on a product or device.
Amounts of Ethylene Oxide residuals present on product post sterilization.
Population of viable microorganisms on or in product and/or sterile barrier system.
Validation or requalification to determine the presence or absence of microorganisms on product.
Population and identification of microorganisms present on a product or device.
Amounts of Ethylene Oxide residuals present on product post sterilization.
Determination of seal strength and integrity of seals used in packaging.
Evaluation of the toxicity of medical devices, products, and materials.
Evaluation of products for the presence of endotoxins, like gram-negative bacteria.
Determination of seal strength and integrity of seals used in packaging.
Evaluation of the toxicity of medical devices, products, and other materials.
Evaluation of products for the presence of endotoxins, like gram-negative bacteria.
Our Expertise
Feel secure with your testing services.
Ethide brings a wealth of experience having over 50 years in the medical device and allied industries.
Microbiology
- Bioburden Test – Membrane Filtration
- Bioburden Test – Pour Plate Method
- Test for aerobic, anaerobic, spore formers and total yeast and molds
- Bioburden recovery rate determinations – Spore inoculation or exhaustive methods
- Biological Indicator population determinations
- Identification of bacteria (IDS System)
- Identification of yeast & mold
- USP Sterility – Product Immersion
- USP Sterility – Membrane Filtration
- USP Sterility – Product Flush
- ANSI/AAMI/ISO Dose Audit – Method One
- ANSI/AAMI/ISO Dose Audit – VDmax Method
- Steerility – Spore Strip Immersion
- Package Integrity – Peel Strength – ASTM Method
- Package Integrity – Accelerated Aging Q10 Coefficient Method
- Shelf Life Sterility – Accelerated Aging / Real-Time expiration dating
- Dye Injection – Leak Test Method
- Vacuum Challenge, Agar Challenge, Closure Challenge Methods
- Viable Air Impactor Samples
- Rodac touch Plate – Surface Samples
- Passive Fall-Out – Settling Plates
- Surface Swab Methods
- Water Analysis / Heterotrophic Plate Count
- Microbial Identification
- On-Site Sample Collection
- Routine Monitoring Programs
Ethide offers a wide range of consulting services to the Medical Device and allied industries. We specialize in sterilization validation protocol development for Ethylene Oxide and Radiation Sterilization processes. Written protocols and complete testing services provide for a consise documented process necessary for regulatory and international requirements. Please contact us for a formal review and estimate for your particular needs.
Toxicology
- Medical Device Extract – Gel Clot w/CSE
- Gel Clot w/RSE
- Inhibition/Enhancement
- Turbidimetric Method
- Chromogenic Method
- Agar Overlay Method – L929 Cells
- MEM Elution Method – L929 Cells
- Direct Contact – L929 Cells
Several USP, ANSI/AAMI/ISO or ASTM In-Vivo test Methods are available to assess the biocompatibility of candidate medical device materials. Please contact our customer service to review applicable tests schemes and schedules.
- Hemolysis Test – Direct Contact
- Hemolysis Test – Extraction method
- (both rabbit blood)
Chemistry
- Ethylene Oxide, Ethylene Chlorohydrin and Ethylene Glycol Determinations
- Simulated – Use Aqueous Extractions
- Exhaustive Extraction Methods
- Determinations of interfering chemicals
Various UV-VIS Spectrophotometry methods are available to determine extractable compounds within elastomers or polymers intended for Medical Device use.
Microbiology
- Bioburden Test – Membrane Filtration
- Bioburden Test – Pour Plate Method
- Test for aerobic, anaerobic, spore formers and total yeast and molds
- Bioburden recovery rate determinations – Spore inoculation or exhaustive methods
- Biological Indicator population determinations
- Identification of bacteria (IDS System)
- Identification of yeast & mold
- USP Sterility – Product Immersion
- USP Sterility – Membrane Filtration
- USP Sterility – Product Flush
- ANSI/AAMI/ISO Dose Audit – Method One
- ANSI/AAMI/ISO Dose Audit – VDmax Method
- Steerility – Spore Strip Immersion
- Package Integrity – Peel Strength – ASTM Method
- Package Integrity – Accelerated Aging Q10 Coefficient Method
- Shelf Life Sterility – Accelerated Aging / Real-Time expiration dating
- Dye Injection – Leak Test Method
- Vacuum Challenge, Agar Challenge, Closure Challenge Methods
- Viable Air Impactor Samples
- Rodac touch Plate – Surface Samples
- Passive Fall-Out – Settling Plates
- Surface Swab Methods
- Water Analysis / Heterotrophic Plate Count
- Microbial Identification
- On-Site Sample Collection
- Routine Monitoring Programs
Ethide offers a wide range of consulting services to the Medical Device and allied industries. We specialize in sterilization validation protocol development for Ethylene Oxide and Radiation Sterilization processes. Written protocols and complete testing services provide for a consise documented process necessary for regulatory and international requirements. Please contact us for a formal review and estimate for your particular needs.
Toxicology
- Medical Device Extract – Gel Clot w/CSE
- Gel Clot w/RSE
- Inhibition/Enhancement
- Turbidimetric Method
- Chromogenic Method
- Agar Overlay Method – L929 Cells
- MEM Elution Method – L929 Cells
- Direct Contact – L929 Cells
Several USP, ANSI/AAMI/ISO or ASTM In-Vivo test Methods are available to assess the biocompatibility of candidate medical device materials. Please contact our customer service to review applicable tests schemes and schedules.
- Hemolysis Test – Direct Contact
- Hemolysis Test – Extraction method
- (both rabbit blood)
Chemistry
- Ethylene Oxide, Ethylene Chlorohydrin and Ethylene Glycol Determinations
- Simulated – Use Aqueous Extractions
- Exhaustive Extraction Methods
- Determinations of interfering chemicals
Various UV-VIS Spectrophotometry methods are available to determine extractable compounds within elastomers or polymers intended for Medical Device use.
Get One Step Ahead Of Competition
Are you looking for expert guidance, better communication and faster testing turnaround times compared to your current contract testing partners? Or are you simply looking for relevant testing strategies? You have come to the right place. We can be your guide and help you navigate complex regulatory pathway for your device.