Is Sensitization Testing Needed For Regulatory Approval Of Medical Devices?

What is Sensitization and hypersensitization?
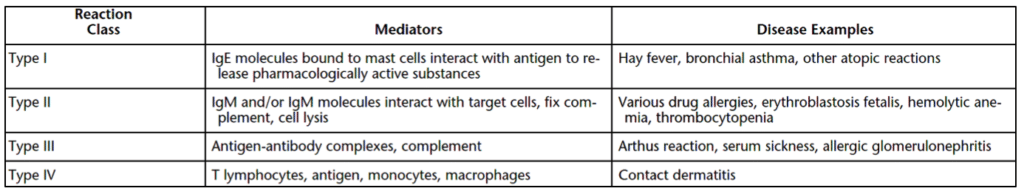
Skin sensitization refers to hypersensitization reactions. There are four types of hypersensitization reactions relevant to medical devices and implants. Descriptions of all hypersensitization reactions and sensitization examples are briefly described here. Type I reactions involve the fixation of immunoglobulin E (IgE) to immune mast cells. This IgE fixation results in the release of histamine, which is the primary cause of allergic reactions. Type II reactions result from immunoglobulin G (IgG ) and immunoglobulin M (IgM) immune cell binding. The body’s complement pathway is activated from the IgM and IgG binding, resulting in pain, swelling, and redness. The complement system results in the killing of cells such that the cellular content is spilled into the body. Type III reactions are caused by antigen-antibody complexes that cause blockages to organs (such as the kidneys). Delayed hypersensitivity reactions are type IV reactions. Type IV reactions are caused by T-cell activation to medical device antigens. Symptoms of hypersensitivity are similar to an allergic reaction and include redness (erythema), pain, and swelling (edema). For this reason, a skin sensitization reaction can also be known as a skin sensitization allergy. All in all, there are multiple ways a sensitivity reaction can be triggered in the human body. Table 1 below summarizes these Gell & Coombs classification types, the immune system mediators of these reactions, and examples of diseases that create these types of hypersensitization.
What is sensitization testing?
Sensitization tests are in vivo tests that evaluate the ability of leachables and unknown antigens to cause hypersensitivity. The tests are designed to determine if a patient will develop a reaction with repeated exposure to a medical device. A few important factors to consider when selecting a sensitivity test include the type and extent of contact with the body, the chemical composition of the product or materials, the product’s manufacturing process, and the product’s sterilization process.
What tests are used for sensitization testing?
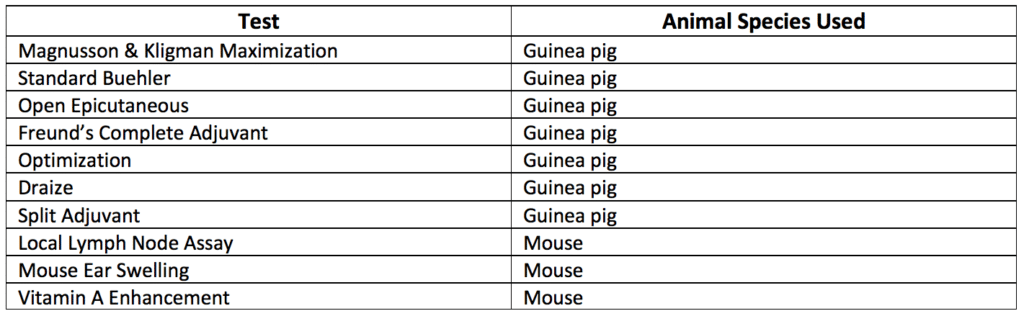
Table 2 summarizes the top nine methods used for regulatory sensitization testing. Most of these tests utilize guinea pigs for sensitivity evaluations. The Magnusson & Kligman Guinea Pig Maximization Test (GPMT) is the gold standard for testing sensitization and sensitization allergy. All other tests are alternatives to the GPMT gold standard. Some sensitization tests evaluate implanted solid articles, while others only evaluate extracts of solid articles. In some medical device and implant applications, toxicology requirements may be satisfied with data available from previously marketed products.
Which medical devices require sensitization testing?
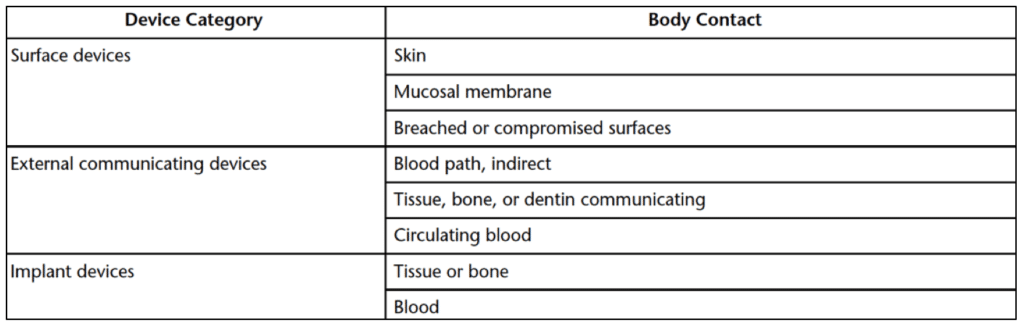
The degree and extent of toxicological testing required are influenced by the type and duration of the bodily contact with a medical device or implant. Devices with human exposure that is limited (less than 24-hours), prolonged (24-hours to 30-days), and permanent (more than 30-days) all require sensitization testing to prevent sensitization allergies. Table 3 below details the categories of medical devices that require sensitization testing. These device categories include surface devices, external communicating devices, and implantable devices.
Summary
Overall, sensitization tests are in vivo tests that evaluate the ability of leachables and unknown antigens to cause hypersensitivity (Type I-IV). The tests are designed to determine if a patient will develop a reaction with repeated exposure to a medical device or implant. Type I sensitization reactions involve IgE and activate histamine. Complement activation defines type II reactions. Type III reactions are caused by antigen-antibody complexes that cause blockages to organs (such as the kidneys). Finally, delayed hypersensitivity reactions are type IV reactions. All sensitivity tests are in-vivo animal studies, and most of these studies utilize guinea pigs for sensitivity evaluations. The Magnusson & Kligman Guinea Pig Maximization Test (GPMT) is the gold standard for sensitization testing. All other tests are alternatives to the GPMT gold standard. In some medical device and implant applications, toxicology requirements may be satisfied with data available from previously marketed products. All in all, ensure you choose a contract testing organization that can support you with appropriate toxicity testing for your unique medical device or product needs.
Ethide Labs is a contract testing organization specializing in Cytotoxicity Testing. Ethide Labs also offers Microbiology Testing, Bioburden Testing, EO Residual Testing, Bacterial Endotoxin Testing, Sterility Testing, Environmental Monitoring & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <1184> Sensitization Testing. Rockville, MD, USA. 2021. (USPC <1184>).
Share this in your social networks


