How does the FDA classify medical devices?
What is the FDA?
The United States Food and Drug Administration (FDA) is a regulatory agency run by the federal Department of Health and Human Services. The FDA protects public health in the United States by ensuring human and veterinary drugs, medical devices, and biological products are safe and effective for their intended use. Further, the FDA regulates the safety of cosmetics and food supplies.
What are sterilization validations, and why are they important?
Since the sterility of a medical device or product is based on acceptance criteria, the process that a product or device undergoes to become sterile must be validated to prove that sterility acceptance criteria are consistently met. Sterility can be assured only using a validated sterilization process under current good manufacturing practices (cGMP). Sterility cannot be demonstrated by reliance on periodic sterility testing of final products alone. Thus, sterilization validations are tests that accumulate data about a sterilization process and statistically prove that the sterilization process can consistently sterilize medical devices or products under “worst-case scenario” conditions.
What are the FDA classifications for medical devices?
The FDA groups devices into one of three regulatory classes based on the potential risk devices have to human health during intended use. Class I devices present little to no potential harm to humans. Class II devices present more risk than Class I devices. Implantable devices, devices that support or sustain life, or devices with a high risk of illness or injury when used fall under Class III devices. Class I and II devices only require a 510(k) submission. Class III devices require a full premarket approval (PMA) submission for FDA marketing clearance. Some Class I and Class II devices fall under an exemption for 510(k) submissions. All exempt devices are subject to limitations on their exemptions. Information on the limitations of device exemptions can be found under 21 CFR Parts 862-892. Additionally, substantially equivalent Class III devices or Class III devices marketed before the FDA’s 1976 amendments (pre-amendment devices) only require a 510(k) submission instead of a complete PMA. Note that pre-amendment devices with “significant changes” will require a full PMA for marketing approval. In the case of Class III substantially equivalent devices, both the intended use of the device and the indications for use are considered for a 510(k) vs. PMA submission. Further details on Class I, Class II, and Class III devices and the approximate percentage of medical devices in each Class can be found in Table 1 below (reproduced from a presentation by Kimberly Piermatteo).
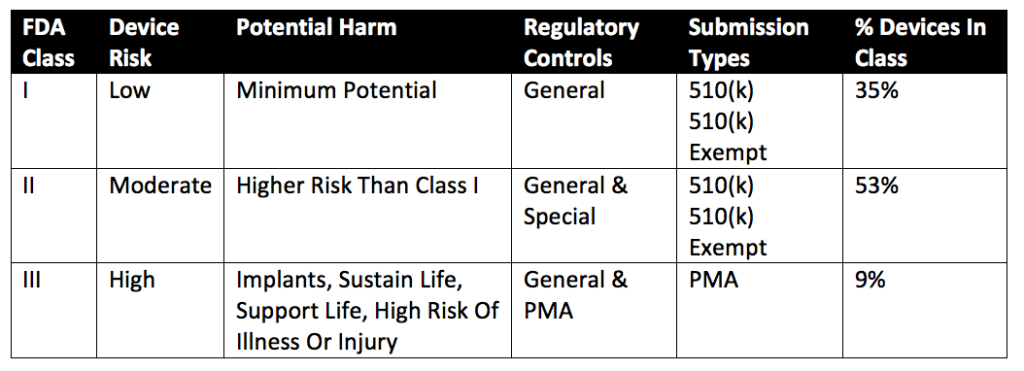
All medical devices must meet Current Good Manufacturing Practices (cGMPs), also known as Quality System Regulation (21 CFR 820). Special Class I and Class II devices are exempt from cGMP requirements. However, many cGMP-exempt devices must still comply with regulatory controls. Device exemptions for cGMPs are detailed in 21 CFR 820. You can also find device exemptions by searching the FDA’s product classification database.
What are the 510(k) exemptions for medical devices?
A majority of Class I and special Class II devices are exempt from safety and efficacy 510(k) requirements. Class I and Class II devices exempt from 510(k) requirements can be found here. The FDA limits 510(k) exemptions for some devices. Thus, confirming a device’s exemption status with the FDA as soon as possible is critical to addressing any unanticipated limitations. A useful resource for finding exemption limitations is 21 CFR 862-892. All FDA exemption announcements can be publicly seen in the federal registrar here.
Summary
Since the sterility of a medical device or product is based on acceptance criteria, the process that a product or device undergoes to become sterile must be validated (through a sterilization validation) to prove that sterility acceptance criteria are consistently met. Sterility can be assured only by using a validated sterilization process under current good manufacturing practices (cGMP). Sterilization validations have five stages: process development, installation qualification, operation qualification, performance qualification, and process control. There are nine categories of process controls necessary for appropriately monitoring a validated sterilization process. Calibration, physical measurements, physical indicators, parametric release, special considerations based on sterilization type, change control, preventative maintenance, periodic process reassessment, and personnel training are the nine process controls. Overall, ensure you choose a contract testing organization that can provide appropriate sterilization validations for your unique medical device or product needs.
Ethide Labs is a contract testing organization specializing in Microbiology Testing. Ethide Labs also offers Bioburden Testing, Bacterial Endotoxin Testing, EO Residual Testing, Sterility Testing, Cytotoxicity Testing, Environmental Monitoring & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
Kimberly Piermatteo. How is my medical device classified? CDRH Learn Presentation. United States Food & Drug Administration.
United States Food & Drug Administration. Class I/II Exemptions. Digital Article 2019.
United States Food & Drug Administration. Classify Your Medical Device. Digital Article 2020.
Share this in your social networks


