USP 1111 Guidelines & Limits For Bioburden
What is sterilization?
Sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization is related to the term sterile, which means a complete absence of viable microorganisms or viruses that have the potential to reproduce. Thus, sterile products that undergo sterilization are often chemical (ethylene oxide gas), dry heat, steam, or radiation sterilized. Sterilization kills any microorganisms inside the products obtained during manufacturing. Sterilization occurs after the product is placed in its final packaging for gas, heat, or radiation sterilization. The last sterilization process after manufacturing is known as terminal sterilization. USP 1111 microbial limits and sterilization bioburden testing will be covered in the following article.
Which microorganisms must sterilization processes eliminate?
Data for microbial growth, survival, and death kinetics comes from work performed in laboratory conditions. However, microorganisms found in manufacturing environments are commonly under nutritional, chemical, dehydration, or other stress that laboratory conditions do not simulate. Therefore, known microbial data may not be as predictive of microbial growth, survival, and death kinetics for microbes found in manufacturing environments. Three primary microbe types require appropriate sterilization (elimination) before a medical product is used: bacteria, fungi (yeast and mold), and viruses.
What is bioburden?
The “bio” in bioburden refers to live biological organisms, and the “burden” in bioburden refers to the concentration of the viable biological organisms. Thus, bioburden is the concentration or quantity of microorganisms in a given area or from a particular sample. In other words, bioburden is the initial population of a microorganism before a sample or item is sterilized. The higher the concentration of viable organisms on a device or product, the higher the burden is to kill those organisms, whether it is killing the organisms through sterilization procedures or killing the organisms through the effort of the human immune system. Bioburden is measured in colony-forming units (CFU). Sterilization bioburden testing and bioburden testing in general is governed by USP 60, USP 61, and USP 62.

What are USP 1111 Microbial limits?
Certain microorganisms in nonsterile preparations can reduce or inactivate the therapeutic activity of the product. Further, disease-causing organisms can cause patient illness if someone is accidentally exposed to them. Thus, manufacturers must ensure that final products contain low (negligible) bioburden. Low bioburden is obtained by implementing Good Manufacturing Practices during pharmaceutical formulation manufacturing, storage, and distribution. USP 1111 details the acceptance criteria for nonsterile pharmaceutical products based on total aerobic microbial count (TAMC) and the total combined yeasts and molds count (TYMC). TAMC and TYMC values are detailed in Table 1 and Table 2 below. For acceptance criteria, a value of 101 CFU means that the maximum acceptable count = 20. A value of 102 CFU means that the maximum acceptable count is 200. Further, a value of 103 CFU means that the maximum acceptable count is 2000.
Table 1 includes a list of microorganisms for which bioburden acceptance criteria (USP microbial limits) are set. The microorganism list in Table 1 isn’t exhaustive. Thus, it is likely that testing for other microbes (outside of Table 1) will be needed to ensure product safety. Alternative tests for microorganisms will depend on the bioburden of starting materials and the manufacturing process.
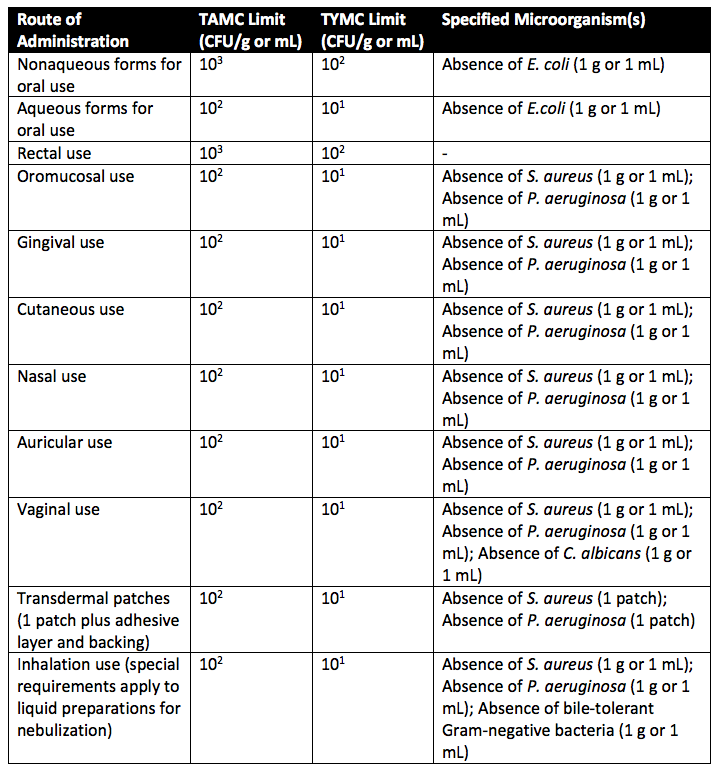

As you can see in Table 2 above, the United States Pharmacopeia (USP) Chapter 1111 requires a USP microbial limit of not more than one thousand colony forming units per gram (or milliliter) for raw materials, excipients, and bulk drug substances.
Any recovered microorganisms (other than Table 1) should be evaluated for:
- Any hazard based on the product’s route of administration
- Whether the product would support the growth of the organism if exposed
- The method of product application
- The intended recipient of the product (risk varies depending on age and preexisting conditions)
- Likelihood of use of immunosuppressive agents or corticosteroids in the intended patient population
- The presence of disease, wounds, or organ damage for intended patients
Summary
Overall, sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization for medical devices and products is critical for ensuring patient safety during product use. There are three primary microbe types that require appropriate sterilization (elimination) before medical product usage: bacteria, fungi (yeast and mold), and viruses. Bioburden is the initial population of a microorganism before a product or item is sterilized.
USP 1111 provides bioburden limits (in colony forming units) for different types of therapeutic applications. Nonsterile dosage form limits vary based on the application. However, all nonsterile dosage forms for pharmaceutical use must have an aerobic microbial bioburden limit of not more than one thousand colony forming units per gram (or milliliter) for raw materials, excipients, and bulk drug substances. All in all, ensure you choose a contract testing organization that can provide appropriate bioburden testing and sterilization validations for your product needs.
Ethide Labs is a contract testing organization that specializes in Sterilization Validations and Bioburden Testing. Ethide Labs provides in-vitro cytotoxicity tests in-house and outsources in-vivo cytotoxicity work for toxicity testing of medical devices, products, and drugs. Ethide Labs also offers Microbiology Testing, Bacterial Endotoxin Testing, EO Residual Testing, Environmental Monitoring, Package Integrity Testing & Cytotoxicity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
International Organization for Standardization. Sterilization of health care products- Moist heat- Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices. Geneva (Switzerland): ISO; 2006. (ISO 17665-1:2006/(R)2016).
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <1111> Microbiological Examination Of Nonsterile Products: Acceptance Criteria For Pharmaceutical Preparations And Substances For Pharmaceutical Use. Rockville, MD, USA. 2021. (USPC <1111>).
United States Pharmacopeial Convention. <1115> Bioburden Control of Non-Sterile Drug Substances and Products. Rockville, MD, USA. 2021. (USPC <1115>).
United States Pharmacopeial Convention. <1116> Microbiological Control & Monitoring of Aseptic Processing Environments. Rockville, MD, USA. 2021. (USPC <1116>).
United States Pharmacopeial Convention. <1211> Sterility Assurance. Rockville, MD, USA. 2021. (USPC <1211>).
Share this in your social networks


