Recovery of Microorganisms For Bioburden Testing
Microorganism recovery efficiency is a determining factor in whether a bioburden test is valid. Microorganism recovery can be performed through repetitive treatment (also referred to as exhaustive recovery) or product inoculation. The preparation of a product sample and the microbial examination method used impact assay design for microorganism recovery for bioburden testing. Additional information on product sample preparation and microbial examination methods is covered below to better understand the subtle complexities of creating an appropriate microorganism recovery assay for bioburden testing.
Special Considerations For Product Sample Preparation
A product’s physical characteristic’s determine how the sample will be prepared for bioburden testing. In unique product cases, an alternative to the procedures described below will be developed.
Water-Soluble Products:
The product is dissolved or diluted for water-soluble products in Buffered Sodium Chloride–Peptone Solution pH 7.0, Phosphate Buffer Solution pH 7.2, or Soybean–Casein Digest Broth.
Non-fatty Products Insoluble in Water:
Non-fatty, water-insoluble products are prepared as a suspension in Buffered Sodium Chloride–Peptone Solution pH 7.0, Phosphate Buffer Solution pH 7.2, or Soybean–Casein Digest Broth. For these products, a surface-active agent (such as polysorbate 80) may be added to assist the suspension.
Fatty Products:
Fatty products are mixed with the minimum quantity of a non-inhibitory, surface-active reagent (such as polysorbate 80) to form an emulsion with Buffered Sodium Chloride–Peptone Solution pH 7.0, Phosphate Buffer Solution pH 7.2, or Soybean–Casein Digest Broth. In some cases, a water bath is used to heat the fatty product and dilutant to not more than 40°C.
Medical Devices:
Medical devices are immersed directly into Buffered Sodium Chloride–Peptone Solution pH 7.0, Phosphate Buffer Solution pH 7.2, or Soybean–Casein Digest Broth for sample preparation.
Fluids or Solids in Aerosol Form:
Aerosol products are transferred into a membrane filter apparatus or a sterile container. Either the total contents or metered doses from each container is tested.
Transdermal Patches:
The release liners of the transdermal patches are removed and placed (adhesive side up) on a sterile tray. The adhesive surface is covered with porous gauze and transferred to a suitable volume of dilutant (i.e., Buffered Sodium Chloride–Peptone Solution pH 7.0, Phosphate Buffer Solution pH 7.2, or Soybean–Casein Digest Broth). The dilutant contains neutralizing agents, such as polysorbate 80 or lecithin, to remove any antimicrobial activity of the transdermal patches. After dilutant immersion, the transdermal patches are shaken vigorously for at least 30 minutes.
Special considerations by Microbial Examination Method
Membrane filtration, pour-plate, surface-spread, and most-probable-number techniques are the most common methodologies used for bioburden testing. Special considerations for using each method in bioburden testing are detailed below.
Membrane Filtration Method:
Membrane filters with a pore size not greater than 0.45 micrometers are used for this method. The filters chosen must not be impacted by the components of the product sample assessed.
A single membrane filter is used for each microorganism inoculated and assayed. An amount equivalent to 1 g or 1 ml of the product sample is filtered through the appropriate membrane filter to capture the organism of interest. The membrane filter is then rinsed with diluent. The membrane filter is then transferred to the surface of Soybean– Casein Digest Agar for total aerobic microbial counts (TAMC) and Sabouraud Dextrose Agar for total yeast and mold counts (TYMC). The plates are incubated under appropriate times and temperatures for each microorganism assessed. Then TAMC and TYMC counts are performed.
Pour-Plate Method:
A milliliter (mL)of prepared sample is added to Petri dishes with 15 to 20 mL of Soybean–Casein Digest Agar or Sabouraud Dextrose Agar. For each microorganism, At least two Petri dishes are used for each organism used for sample inoculation. After inoculation, the plates are incubated under appropriate times and temperatures for each microorganism assessed. Following incubation, the arithmetic mean and number of the counts are taken. Then the colony-forming units (CFU) of the original inoculum are calculated.
Surface-Spread Method:
Petri dishes with 15 to 20 mL of Soybean–Casein Digest Agar or Sabouraud Dextrose Agar are allowed to solidify. Plates are dried via sterile laminar airflow, and a volume of at least 0.1 mL of sample is spread over the Petri dish’s surface. At least two Petri dishes are used for each microorganism used for sample inoculation. Incubation times and counting methods for the Surface-Spread Method are the same as those for the Pour-Plate Method.
Most-Probable-Number (MPN) Method:
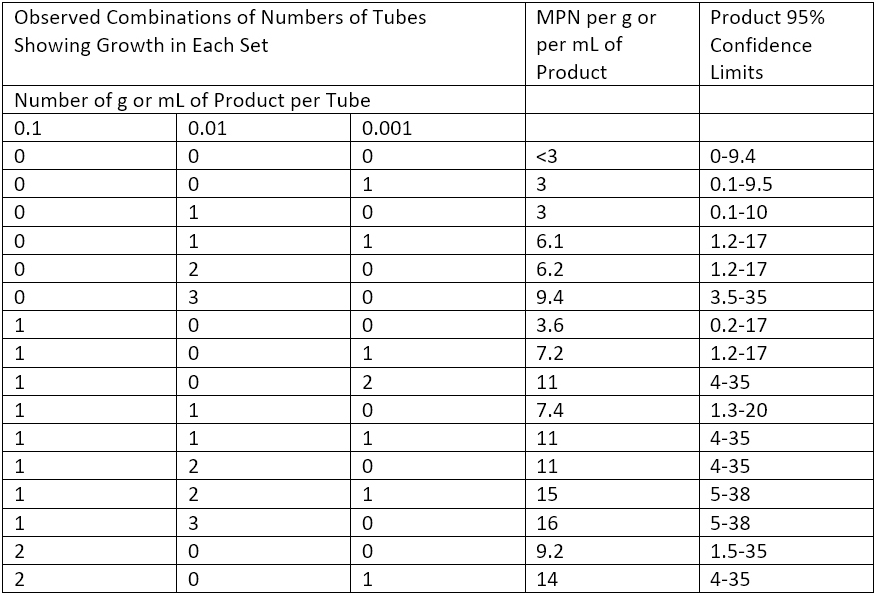
The MPN Method has less precision and accuracy than Membrane Filtration or the Plate-Count Methods, especially when it comes to evaluating molds. However, in cases of low bioburden content (or when no other method is available), the MPN Method is used. Three serial dilutions of the product sample are prepared for the MPN Method. A milliliter of each serial dilution is added to tubes with 9 to 10 mL of Soybean–Casein Digest Broth in triplicate. The nine tubes are then inoculated with specific microorganisms and incubated at 30° to 35°C for not more than three days. If results are challenging to read, subculture the samples for an additional 1 to 2 days at the same temperature, and use these results. The most probable number of microorganisms per gram or mL of the product to be examined is determined using Table 3 of the USP 61, reproduced in part below as Table 1.
Summary
Overall, microorganism recovery efficiency is essential for bioburden test validity. The preparation of a product sample and the microbial examination method used impact assay design for microorganism recovery of bioburden testing. Product sample preparation techniques for water-soluble products, non-fatty products, fatty products, medical devices, transdermal patches, and aerosolized products are described in this article. Additionally, this article describes Membrane Filtration, Pour-Plate, Surface-Spread, and MPN Methods for microbial examination. All of these microbial examination methods and product sample preparations indicate the subtle complexities of creating an appropriate microorganism recovery assay for bioburden testing of your medical device or product.
Ethide Labs is a contract testing organization that specializes in Bioburden Testing. Ethide Labs also offers Bacterial Endotoxin Testing, Environmental Monitoring, Sterilization Validations, Microbiology Testing, EO Residual Testing, Package Integrity Testing & Cytotoxicity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <61> Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests. Rockville, MD, USA. 2021. (USPC <61>).
Share this in your social networks