Bioburden vs. Biofilms For Medical Device Testing
What is bioburden?
The “bio” in bioburden refers to live biological organisms, and the “burden” in bioburden refers to the concentration of the viable biological organisms. Thus, bioburden is the concentration or quantity of microorganisms in a given area or from a particular sample. The higher the concentration of viable organisms on a device or product, the higher the burden is to kill those organisms, whether it is killing the organisms through sterilization procedures or killing the organisms through the effort of the human immune system.
What are biofilms?
Biofilms are microbial communities created from the attachment and growth of different microbes onto something. In the case of medical devices, biofilms attach to the surface of medical implants such as sutures, catheters, stents, and other implants. Biofilms are like cities for bacteria, yeast, and other organisms. In these “cities,” the microbes are “people.” The biofilm “city” structure is formed from the extracellular matrix, exopolysaccharides, and extracellular DNA components produced by the microbes. Biofilms, once produced, are extremely difficult to remove. Biofilms are difficult to eliminate because the biofilm protects the microorganisms living there. The safety biofilms provide includes protection from altered pH, osmolarity, starvation, antibiotics, immune cells, and mechanical shear forces.
How do bioburden and biofilms affect medical device safety?
Medical devices are sterilized to reduce the patient’s microbial exposure during surgery or medical treatment. While the bioburden on a medical device can essentially be eliminated with sterilization or aseptic techniques, microbes from the patient can still attach to devices used during surgery (or the implantable devices themselves) and cause biofilm formation on the implanted device over time. This biofilm formation can be destructive, resulting in patient illness and removal of the implanted device. Thus, the bioburden of the product and the bioburden of the patient both affect the risk of biofilm formation on medical devices and, ultimately, the risk of post-treatment patient complications.
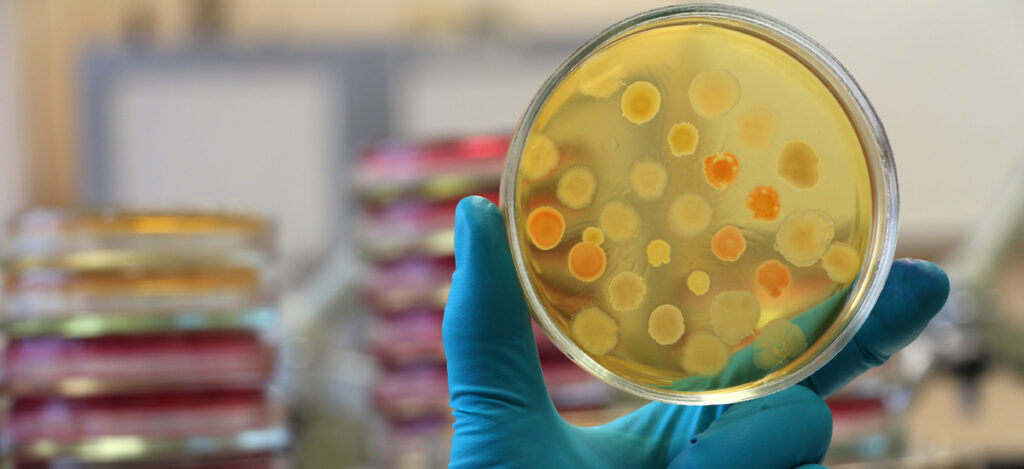
What is a bioburden test?
Bioburden testing measures the viable organisms present in a sample. Viable organisms include spores, bacteria, yeast, and more. The total viable organisms present is expressed as a total viable count. This total viable count (expressed as colony forming units) provides a value for the microbial contamination level within a particular sample. Combined bioburden and microbiology testing can identify the specific types of live microorganisms present in a product, package, or manufacturing environment. Bioburden testing follows the methods outlined in USP 60, USP 61, and USP 62.
Why are bioburden testing and the prevention of biofilm formation important for medical devices?
Bioburden testing is an important quality control step that detects the level of contamination of a product at any stage, from initial product manufacture to final distribution. As microorganisms exist on every surface (including our body), bioburden can be accidentally introduced during the manufacturing or packaging process in many ways. Some of the most common examples are contamination through the raw materials used, technicians, tubing/piping used to transfer product between development stages in a process, or the manufacturing environment itself. With such abundant sources of contamination, regular bioburden testing supports the long-term control of manufacturing sites.
Bioburden testing also supports the microbial control of a product before terminal sterilization. Sterilization process parameters are made based on a certain microbial exposure level. Sterilization processes can only sterilize products with a live microbial count that fits within the acceptance criteria for the sterilization process. Thus, bioburden testing and environmental microbial control are vital to sterilization validations and ensuring that the acceptance criteria for the sterilization process are met consistently for each manufactured product.
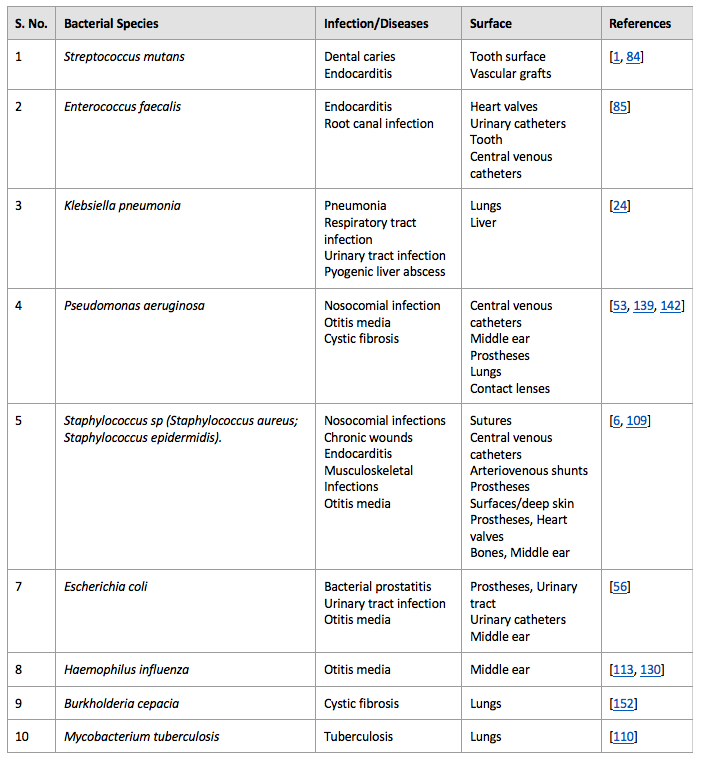
When bioburden limits for the sterilization process or aseptic conditions are exceeded, viable microbes on medical devices and products can create biofilms within the medical device packaging or within patients upon device use. Bacterial biofilms are responsible for approximately 80% of chronic and recurrent infections. Additionally, microbes within biofilms have ten to a thousand times more antibiotics resistance than microbial cells existing outside of a biofilm. Due to their antibiotic resistance, biofilm infections can only be treated by implant removal, which increases the cost of the treatment and is problematic for the patient. Thus, biofilm prevention for medical devices is of utmost concern for patient safety. Some of the common biofilm-associated infections are listed in Table 1 below (reproduced from D. Sharma et al.).
Summary
Overall, testing for bioburden is an imperative safety measure for regulatory approval of medical devices and products. Bioburden testing ensures that medical devices are free of unwanted microbes so that users will not be at risk of biofilm formation or illness following device use or implantation. Ensure you choose a contract testing organization that can support you with appropriate bioburden biocompatibility testing for your unique product needs.
Ethide Labs is a contract testing organization that specializes in Bioburden Testing. Ethide Labs provides in-vitro cytotoxicity tests in-house and outsources in-vivo cytotoxicity work for toxicity testing of medical devices, products, and drugs. Ethide Labs also offers Sterilization Validations, Microbiology Testing, Bacterial Endotoxin Testing, EO Residual Testing, Environmental Monitoring, Package Integrity Testing & Cytotoxicity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
76 Sharma, L. Misba, & A. U. Khan. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrobial Resistance & Infection Control. Volume 8: Article 76. 2019.
United States Pharmacopeial Convention. <60> Microbiological Examination of Nonsterile Products- Tests for Burkholderia Cepacia Complex. Rockville, MD, USA. 2021. (USPC <60>).
United States Pharmacopeial Convention. <61> Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests. Rockville, MD, USA. 2021. (USPC <61>).
United States Pharmacopeial Convention. <62> Microbiological Examination Of Nonsterile Products: Tests For Specified Microorganisms. Rockville, MD, USA. 2021. (USP <62>).
Share this in your social networks


