Importance Of Medical Devices In Healthcare
What are medical devices?
Medical devices cover a vast range of non-pharmaceutical products used for disease treatment, monitoring, or prevention. For example, medical instrumentation (electronics), mechanical devices, and combination devices are all included in the definition of a medical device. Some combination devices have chemical, pharmaceutical, or biological components. Medical devices also cover implantable devices, devices used during surgical procedures, and non-implantable devices used at home by patients. This article will touch upon medical device industry trends, medical device best practices, and medical device industry growth in healthcare.
Why is the importance of medical devices in healthcare relevant?
Medical device creation and usage have risen along with the rise in digital technology. However, the efficacy and safety standards for many medical devices are less strict than for medications. Consequently, novel medical devices are adopted within healthcare systems without adequate assessment of their cost-effectiveness or patient usage benefits. For pharmaceuticals, only the pharmacological effects and clinical efficacy are of interest. However, medical devices provide patient benefits (such as physical, mental, and social well-being) beyond clinical outcomes. Some current medical device industry trends are the rise in 3D printed devices (or device parts), the use of medical robotics, and connectivity increases for medical devices between platforms. Another topic related to medical device best practices is cybersecurity and how to keep medical information safe with increasing device connectivity.
What are some examples of well-known medical devices used in the healthcare industry?
- Glucose monitors
- Pacemakers
- Stents
- Hip and knee implants
- Artificial limbs
- Respirators
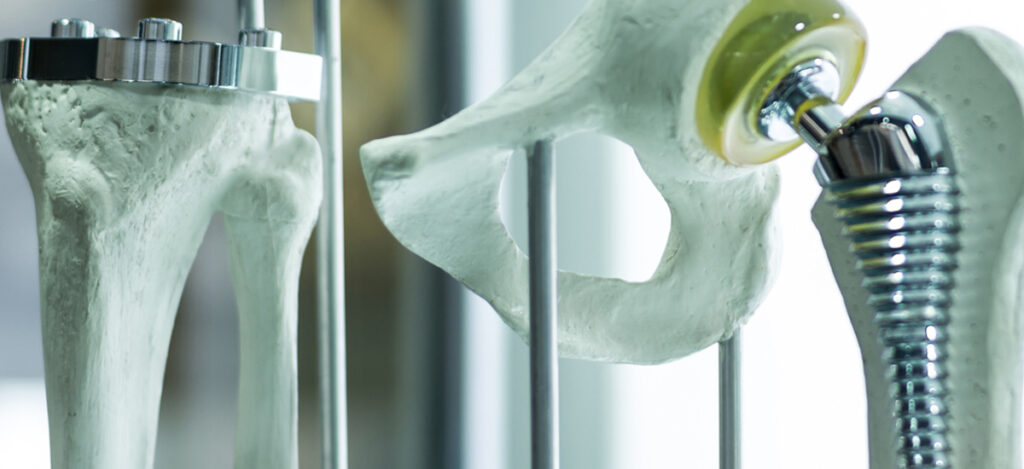
What are the benefits of medical device use, industry growth, & medical device industry trends?
Medical devices provide a variety of benefits to patients and healthcare providers. For patients, medical devices offer reliability, a reduced need for assistance, increased comfort/security, and a sense of control. Medical devices provide efficiency, reliability, automation, human error reduction, and ease of use for healthcare providers. Medical devices used by surgeons can provide improved efficacy, longevity, visibility, agility, communication, and monitoring. Overall, patient-centric medical devices aim to improve patient care, safety, treatment consistency, vials monitoring, or treatment efficacy. Hospital-centric medical devices aim to boost capability, reduce cost, or save time. Technologies following medical device industry trends, such as 3D printing and medical robotics, are examples for devices aimed to boost capability, save time, and reduce cost. Interestingly, an article from McKinsey shared that medical device industry growth for the top 30 medical technology companies was small and underperforming in the S&P despite their size and diversification. Meanwhile, medical device industry growth continues to increase in small-cap and mid-cap companies at a compound annual growth rate (CAGR) of about 10%. Overall, medical device industry trends and medical device industry growth continues to move in an upwards trajectory as our aging population increases and new treatments are available.
What are the patient-related risks to medical device usage?
The medical devices that present the most significant risk to patients are implantable or inserted into the body. With implantable devices, the greatest risk to patients is hospital-associated infection. In the case of devices temporarily inserted into the body, like laryngoscopes, the risk to the patient is primarily physical. In the case of a laryngoscope, the risk is discomfort due to physical abrasion or the results of temporarily cutting off the body’s natural air supply during use. For medical devices used for monitoring, inaccurate results can occur due to improper machine maintenance, setup, or result interpretation. Thus, it is critical to implement regular maintenance schedules so that medical device best practices for monitoring instruments can be met.
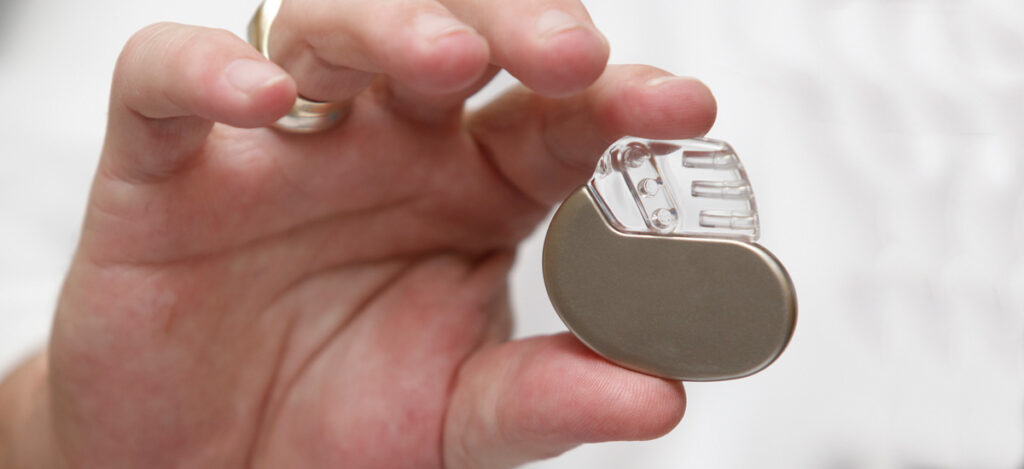
What are the healthcare facility risks to medical device usage?
Healthcare facilities’ primary medical device risks are low proof of efficacy, learning curve effects, dynamic pricing, and transferability. All of these risks are further explained below.
Low Proof Of Efficacy
For pharmaceuticals, randomized controlled trials for efficacy are performed comparing drugs to placebo or gold standard treatment. However, efficacy comparisons to standard care are not required for bringing a medical device to market. Thus, there is a lack of high-quality evidence when comparing the efficacy of various medical devices for use in a healthcare facility. Some healthcare facilities create risk-sharing schemes to support medical device evaluation. Also, with technologies changing so rapidly over time, periodic reassessments taking evolving real-world evidence into account is the best guide for medical device assessment versus a single decision point. Ultimately it isn’t easy to evaluate the cost versus utility and the cost versus quality of life for various technologies.
Learning Curve Effects
Most medical devices are operated by a healthcare professional. Thus, the combined effect of the medical device itself and healthcare professional operation influences patient care. In other words, medical device handling, interpretation, and maintenance all impact patient treatment or preventative care. Medical device operation, interpretation, and maintenance require some level of training. Thus, there is a “learning curve” for optimal medical device use and full clinical benefits. With a learning curve risk, patients that receive treatment or preventative care from healthcare professionals that have not optimized their medical device operation efficacy will not receive full clinical benefits. As of 2021, there is no standardized way to track or evaluate the effects of learning curve risk for medical device operation.
Dynamic Pricing
Complicated medical devices are a high-cost investment up-front. The patient and cost benefits of these devices only show after at least a year of use. Furthermore, many complex medical devices require regular maintenance and calibration for proper function.
Transferability
Due to the learning curves of medical devices and differences between medical device training standards from hospital to hospital, results from medical devices used at one institution may not reflect the results received from another hospital. Thus, the accuracy of data transfer between institutions using the same medical devices is more challenging than organization-to-organization patient results with pharmaceuticals.
Summary
Overall, medical devices cover a vast range of products used to monitor, prevent, and treat various ailments. Medical devices cover medical instrumentation (electronics), mechanical devices, and combination devices. Combination devices have two or more chemical, electrical, mechanical, pharmaceutical, or biological components. Medical devices cover multiple uses, including implantable devices, devices used during surgical procedures, and non-implantable devices used at home by patients. Medical device creation and usage have risen along with the rise in digital technology. However, standards for many medical devices are less strict than for medications. Consequently, novel medical devices are adopted within healthcare systems without adequate assessment of their cost-effectiveness or patient usage benefits. Indeed, the introduction of a novel medical device comes with a host of risks for healthcare facilities. These risks include insufficient proof of efficacy, learning curve effects, dynamic pricing, and transferability. When designing a medical device, information on the best training practices for the device and how that device compares to gold standards for treatment, preventative care, or patient monitoring is influential in adopting the use of your medical device. Further, during medical device development, ensure you choose a contract testing organization that can support you with appropriate biocompatibility and package integrity testing for your unique medical device needs.
Ethide Labs is a contract testing organization specializing in Microbiology Testing. Ethide Labs also offers Bioburden Testing, Bacterial Endotoxin Testing, EO Residual Testing, Sterility Testing, Cytotoxicity Testing, Environmental Monitoring & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
Eva Lesén, Ingela Björholt, Anders Ingelgård and Fredrik J. Olson. Exploration And Preferential Ranking Of Patient Benefits Of Medical Devices: A New And Generic Instrument For Health Economic Assessments. Cambridge University Press. October 2017.
Maximilian Blüher, Sita J. Saunders, Virginie Mittard, Rafael Torrejon Torres, Jason A. Davis and Rhodri Saunders. Critical Review of European Health-Economic Guidelines for the Health Technology Assessment of Medical Devices. Frontiers In Medicine. November 2019.
McKinsey & Company. Accelerating growth in medtech: The next surge in portfolio moves. Article. May 20, 2022.
Share this in your social networks


