Neutralization of Antimicrobial Activity
What is antimicrobial activity?
Components of medical products or coatings placed upon medical devices or materials can have antimicrobial effects. In other words, these coatings or components are able to slow the growth of microbes such as bacteria, yeast, or molds. This ability to slow the growth of microbes is known as antimicrobial activity.
Why is the neutralization of antimicrobial activity important for bioburden testing?
While antimicrobial activity is often advantageous when producing a medical product or device, it can get in the way of accurate bioburden testing for regulatory approval. This is because antimicrobial activity can give your device an advantage over products that do not have antimicrobial activity when it comes to bioburden testing. Thus, to determine if your product meets regulatory standards in the traditional sense, the antimicrobial activity of the samples from your product or device will need to be neutralized. You may not know your product or device has antimicrobial activity in some instances. In this case, you will need to run bioburden tests on your original product sample and on product samples that have antimicrobial activity neutralized.
How do you neutralize the antimicrobial activity of a product sample?
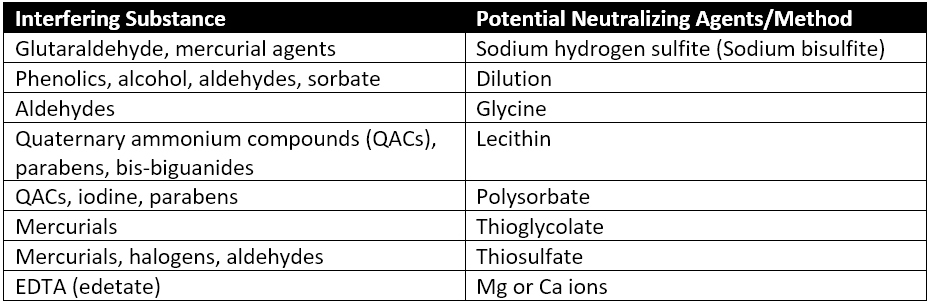
There are cases where the antimicrobial activity of a product sample is unknown. In this case, if microbial growth is reduced by a factor greater than 2, steps must be taken to reduce the antimicrobial activity of the product samples. The three most common ways to neutralize the antimicrobial activity of your product samples are diluting your samples, incorporating neutralizing agents into your test specimens, or performing membrane filtration of your samples to remove antimicrobial agents. Many times, neutralization will combine all three techniques to prepare your specimens for bioburden testing. Common neutralizing agents from Table 2 of USP 61 are reproduced in Table 1 below.
Neutralizing agents can be added to a diluent or culture medium to prevent the activity of the antimicrobial agents. These neutralizing agents may bind to antimicrobial agents directly to inhibit antimicrobial activity via other mechanisms. If neutralizing agents are used, bioburden testing must use a control sample with the neutralizer without the product sample to ensure that the neutralizing agent isn’t toxic to the microorganisms used for bioburden testing. Inactivators used for neutralization must be effective and non-toxic to the organisms tested. Any surface-active agents used for neutralization cannot be toxic to the microorganisms tested and must be compatible with any inactivators used in sample preparation.
In rare cases where a suitable neutralizing method cannot be found, the failure to detect the growth of the microorganism during bioburden testing is assumed to be due to the microbial activity of the product. In this case, the product under assessment is deemed resistant to contamination with the specific microorganism evaluated. Since it is possible that the product only inhibits particular strains of the organism assessed, an additional test is performed with the highest dilution factor of the product sample that is compatible with microbial growth and the specific acceptance criterion.
Summary
While antimicrobial activity can assist in preventing patient infections when a medical product or device is in use, antimicrobial activity can get in the way of accurate bioburden testing for regulatory approval. Luckily, there are methods to neutralize your product samples so that you can meet bioburden testing criteria for your product. The three most common ways to counteract the antimicrobial activity of your product samples are diluting your samples, incorporating neutralizing agents into your specimens, or performing membrane filtration of your samples to remove antimicrobial agents. In some instances, sample preparation will combine all three techniques to prepare your samples for bioburden testing. All in all, meeting bioburden testing criteria is a crucial step towards getting your device regulatory approval.
Ethide Labs is a contract testing organization that specializes in Bioburden Testing. Ethide Labs also offers Bacterial Endotoxin Testing, Environmental Monitoring, Sterilization Validations, Microbiology Testing, EO Residual Testing, Package Integrity Testing & Cytotoxicity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <61> Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests. Rockville, MD, USA. 2021. (USPC <61>).
Share this in your social networks