F Value Calculations For Medical Device & Product Sterilization
What is sterilization?
Sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization is related to the term sterile, which means a complete absence of viable microorganisms or viruses that have the potential to reproduce. Thus, sterile products that undergo sterilization are often sterilized through radiation, chemicals, or heat. Sterilization kills any microorganisms inside the products obtained during manufacturing. Sterilization occurs after the product is placed in its final packaging for dry heat sterilization, steam sterilization, ethylene oxide sterilization, vapor sterilization, liquid phase sterilization, and radiation sterilization. The last sterilization process after manufacturing is known as terminal sterilization. Please see our sterilization comparison chart for more information on which sterilization method is best for your medical device.
What are microbial growth and death kinetics?
Microbial growth is exponential. When low-level contamination occurs, it cannot be identified immediately. There is a lag time before the contaminating microbes have grown to the point where they can be measured and verify contamination took place. This lag phase varies between minutes to years depending upon the microbial species, product storage temperature, available nutrients, and other factors that impact microbial growth kinetics. For sterilization, relevant lag times are on the scale of minutes to hours. Once growth starts, it progresses quite rapidly. For example, a typical bacterial cell can produce over 1,300,000 cells in 8 hours! Eventually, microbial growth plateaus to a stationary phase when critical nutrients are depleted, oxygen diffusion is inhibited, or toxic metabolites accumulate. The approximate population where stationary phases exist is 10,000,000 cells/milliliter.
Microbial death kinetics, like growth kinetics, are exponential. Figure 17-2 below is an example of a microbial death kinetic plot. Microbial death plots can be used to calculate values and constants that describe microbial death kinetics for organisms. The most common values and constants used in microbial death kinetic studies are bioburden (the initial microbial population), D value, Z value, and F value. The relationship between Bioburden, D value and Z value can be found HERE.
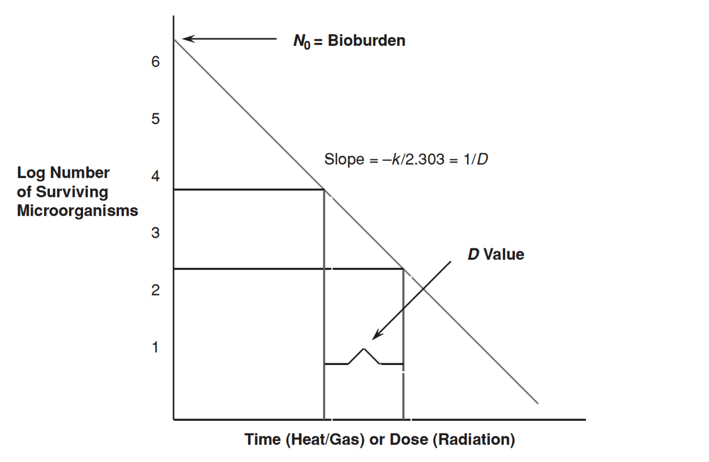
What is an F value, and how is an F value calculated?
The F value is the time (at a given temperature) that a product receives a lethal amount of sterilization. In other words, the F value is a measure of sterilization lethality per unit time. Sterilization lethality is often determined using biological indicators like bacterial spores. In rare cases, dry heat sterilizations also use F values. However, the F value is almost exclusively applied to steam sterilization to determine minimum and overkill cycles for terminal sterilization validation processes. In contrast to D values, the F value is not clock time but “equivalent time.” Equivalent time is dependent upon the temperature. For example, if the actual temperature measured (T) is lower than the reference temperature (T0), the equivalent time is always shorter than the actual one. Z is the thermal resistance value calculated from D values at various temperatures in the F value equation below. Z values are also called lethality values. ∆t is the time interval between temperature determinations.
F values are calculated using the following equation:
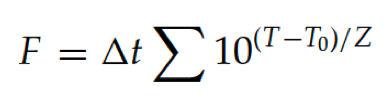
The F value relates microbial death efficiency (lethality) of a given temperature to a standard temperature known to kill microbes. For example, an F value of eight minutes means that the item sterilized was exposed to a sterilization process equivalent to eight minutes at the reference temperature (e.g., 121◦C) regardless of actual temperatures attained.
Table 17-3 below demonstrate the data used to calculate F values. Table 17-3 displays time and temperature data along with calculations for the exponential relationship between actual temperature, reference temperature (121◦C), and a Z value of 10◦C. Taking the sum of the calculated exponential relationships yields a total of 8.522 minutes. Since the change in t (time between data collection) is one minute, the F value for the data in Table 17-3 is 8.522.
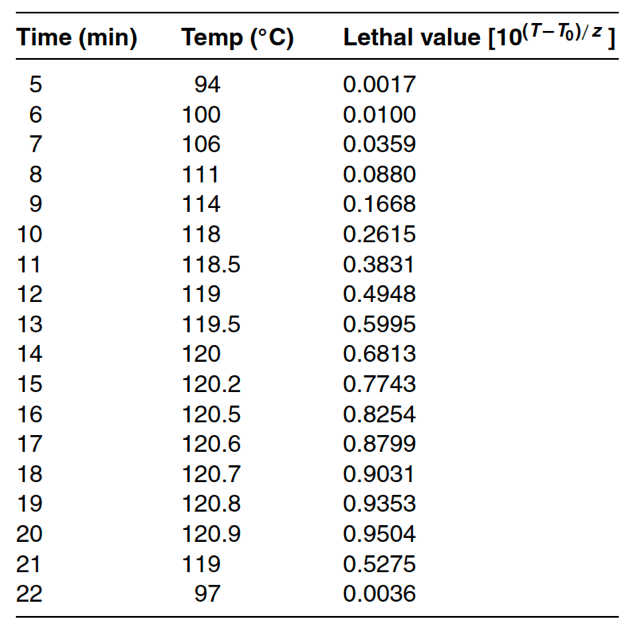
Figure 17-4 of real sterilization temperatures over time compared to equivalent sterilization time visually shows how F value equivalency works. In Figure 17-4, actual temperature versus time data during a sterilization cycle and the calculated exponential term at a given time interval are plotted. The F value result is the area under the time-temperature curve (the darkened area). In application to sterilization validations and optimization, the sterilization cycle temperature and time are manipulated to produce a cycle with the desired F value. Such temperature and time manipulations allow for sterilization cycles to be uniquely tailored so that products are sterilized to the appropriate F value without compromising product integrity.
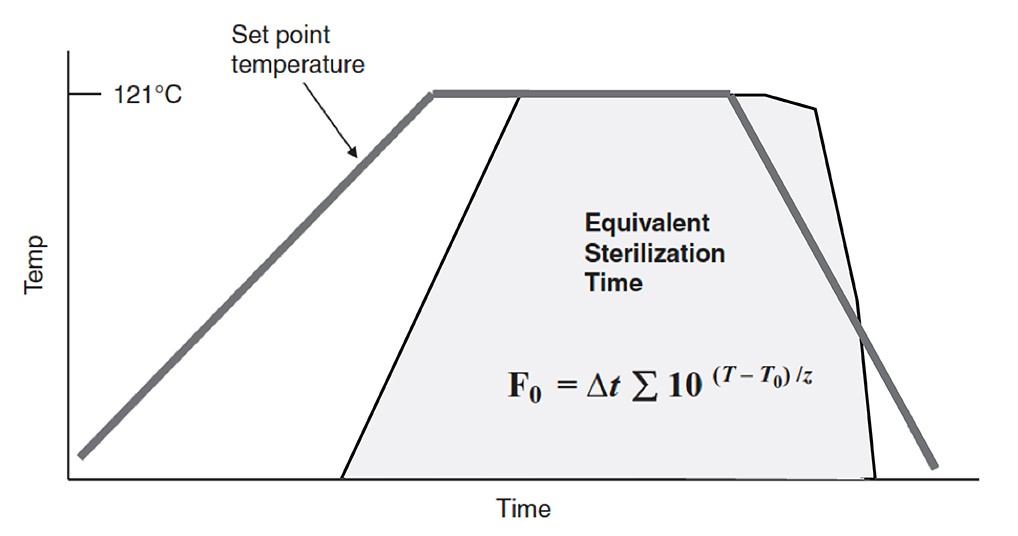
Table 17-2B below depicts the effect of common sterilization methods on the D value of the same microorganism (B. subtilis var. niger). As you can see, the effectiveness of common microbial treatments varies greatly depending upon the microorganism being eradicated. Over time, microbial D values have increased, representing the tendency of microbial life to develop resistance to the methods used to destroy them.
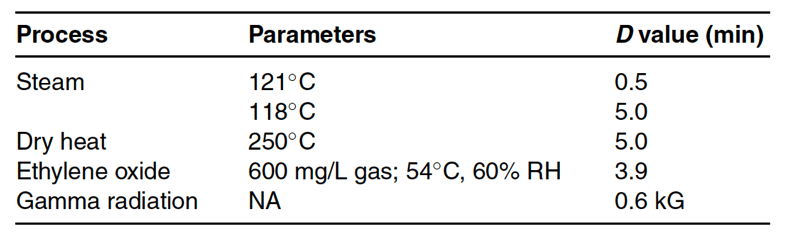
F values can also be calculated using the biological equation (for FT) described in Figure 17-5 below.
In this equation, N0 and Ns are the initial and final microbial populations, respectively. The biological F value calculates what F value is required to obtain a spore log reduction value as a function of the D value of the specific spore. Spores are more resistant to sterilization since they are in a state of hibernation. As an example of a biological F value calculation, if the D value is known to be two minutes and a 12-log reduction in that spore indicator organism is required for sterilization, the minimum F value required is 24 minutes.
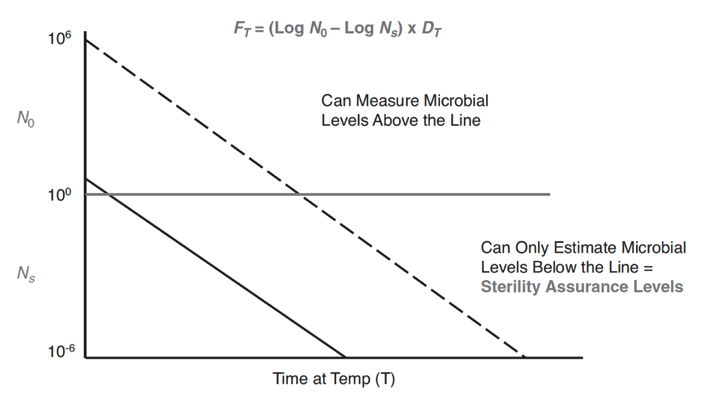
Figure 17-5 above shows a plot of the microbial population versus time at a given temperature (a D value graph). As microbial death is logarithmic, it never hits zero. Since microbial death never hits zero, there are areas where microbial death is at undetectable levels. Undetectable microbial growth levels are why sterilization is completed to a sterility assurance level (SAL). An example industry SAL value is 10-6, also known as the probability of one non-sterile item in a million. The higher the initial microbial concentration, the longer it will take to reach a particular SAL. Thus, using environmental monitoring techniques to reduce the initial microbial concentration on products and devices supports faster sterilization to the desired SAL. Overkill sterilization cycles use plots like Figure 17-5 to calculate the time required to achieve a certain SAL or microbial log reduction. As an example of SAL calculation, if the initial microbial contamination of the sample were 10 and the sterilization cycle produced a 12-log reduction of that organism, then the SAL would be 10−13 (a probability of one unit out of ten billion sterilized units would be contaminated).
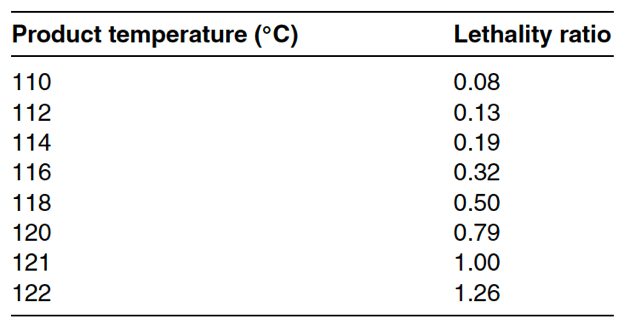
Table 17-4 below displays Z values versus temperature. In this table, you can see that the lethal effect of one-minute exposure at 118◦C is half the lethality of a one-minute exposure at 121◦C. As the temperature falls away from 121◦C, the ability to kill microbes exponentially decreases. When temperature increases from 121◦C, the lethal effect exponentially increases. These temperature relationships from F value calculations can support designing optimal temperatures for sterilization cycles.

Summary
Overall, sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization for medical devices and products is critical for ensuring patient safety during product use. Steam sterilization is the most common form of product sterilization. F values are a measure of sterilization lethality per unit time. F values can be calculated using a sum of the exponential relationship between the actual temperature and reference temperature with Z values. F values can also be calculated using a biological equation that determines the F value required to obtain a spore log reduction value as a function of the D value of the specific spore. F values are valuable for the sterilization cycle and sterilization validation optimization for desired sterility assurance levels. Ensure you choose a contract testing organization that can provide appropriate sterilization validations for your product needs.
Ethide Labs is a contract testing organization that specializes in Sterilization Validations. Ethide Labs also offers Bioburden Testing, Microbiology Testing, Bacterial Endotoxin Testing, EO Residual Testing, Environmental Monitoring, Package Integrity Testing & Cytotoxicity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
International Organization for Standardization. Sterilization of health care products- Moist heat- Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices. Geneva (Switzerland): ISO; 2006. (ISO 17665-1:2006/(R)2016).
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
United States Pharmacopeial Convention. <1115> Bioburden Control of Non-Sterile Drug Substances and Products. Rockville, MD, USA. 2021. (USPC <1115>).
United States Pharmacopeial Convention. <1116> Microbiological Control & Monitoring of Aseptic Processing Environments. Rockville, MD, USA. 2021. (USPC <1116>).
United States Pharmacopeial Convention. <1211> Sterility Assurance. Rockville, MD, USA. 2021. (USPC <1211>).
Share this in your social networks


