Why Are Bacterial Spores Hard To Sterilize?
What is sterilization?
Sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization is related to the term sterile, which means a complete absence of viable microorganisms or microbes that have the potential to reproduce. Thus, sterile products that undergo sterilization are often chemically or heat sterilized after being placed in their final packaging. The chemical or heat sterilization kills any microorganisms inside the products (obtained during manufacturing and packaging). This chemical or heat sterilization process after final product packaging is known as terminal sterilization.
Four main methods sterilize items:
- Heat
- Gas
- Radiation
- Filtration
Another way to classify these four sterilization methods is:
- Thermal
- Moist
- Dry
- Nonthermal
- Filtration
- Radiation
- Chemical
- Gaseous
- Liquid
Which microorganisms must sterilization processes eliminate?
Sterilization processes eliminate three primary microbe types: bacteria, fungi (yeast and mold), and viruses.
What are bacterial spores?
Bacterial spores only develop from some species of bacteria, gram-positive bacteria. Gram-positive bacteria differ from gram-negative bacteria in that they do not contain an outer cell wall. Examples of common gram-positive bacteria are Staphylococcus, Streptococcus, Bacillus, and Clostridium. Bacterial spore formation occurs when stressed bacterial cells develop an outer shell to protect themselves from adverse environmental conditions such as heat, chemicals, and nutrient depletion. The most common spore formers are Bacillus and Clostridium species. Note that these common spore formers are gram-positive bacteria. The issue with bacterial spore forms is that spore forms are hundreds of times more difficult to destroy than traditional bacteria. Thus, using spore-forming bacteria (or equivalents) as biological indicators is critical to validate sterilization processes. Indeed, if a sterilization process can kill certain concentrations of spore-forming bacteria effectively, the sterilization process will also be able to kill other resident bacteria.
Why are bacterial spores hard to kill?
Bacterial spore formation is a coping mechanism for cells experiencing harsh environmental conditions. Spore formation is a form of protection for bacterial cells that conserves the energy of the cell. As the spore state is a state of energy conservation (hibernation), bacterial spores are a dormant form of bacteria. They have minimal metabolism, low respiration, and reduced enzyme production. Gram-positive bacteria are best known for forming spores. Spores form at multiple locations within a spore-forming bacterial cell. These intracellular spores are called endospores.
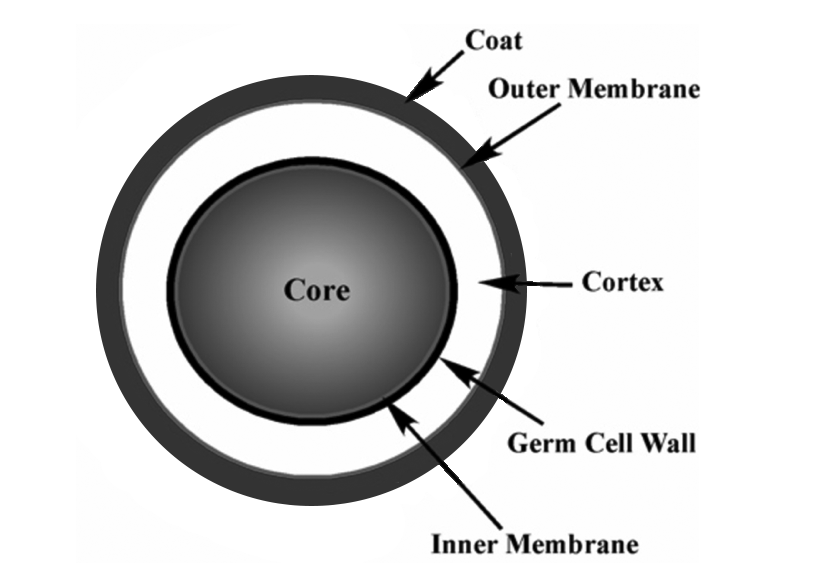
Figure 1 depicts the thick-walled proteinaceous outer coating of the endospore, which surrounds the endospore’s cortex and core. This proteinaceous coat provides most of the endospore’s protection against chemicals and enzymes. The cortex beneath the endospore’s coat is a thick layer of peptidoglycan. The cortex provides dehydration for the spore core and allows the endospore to survive high temperatures. Under the cortex is a germ cell wall that will become the bacterium’s cell wall after the endospore germinates. The core at the center of the endospore houses the cell’s DNA and ribosomes. The core also contains small acid-soluble proteins that support the endospore in resisting harm when exposed to UV light or DNA-damaging chemicals.
Endospore formation takes several hours. The first step is an asymmetric division of the bacterial cell that creates a mother cell and a forespore. The forespore is then engulfed by the mother cell to form a cell within a cell. Next, the cortex is formed around the forespore core, followed by the formation of the proteinaceous coat around the cortex. Finally, dehydration and maturation of the endospore occurs, followed by the mother cell initiating programmed cell death. The endospore then remains dormant until favorable environmental conditions are met.
As mentioned earlier, endospores are extremely difficult to kill as they will survive temperatures up to 150°C and as low as near absolute zero. Endospores are resistant to chemical agents (including alcohol), ultraviolet radiation, extreme pH gradients, drought, and nutrition depletion. When the surrounding environment favors bacterial growth, endospores leave their dormant state and germinate back into an active bacterial cell with normal metabolism. There are two approaches to killing bacterial spores. The first is that the environmental conditions must stimulate the bacterial spore cells back to their active bacterial state so that they can be killed using traditional sterilization techniques. Triggers for spore germination back to an active state include temperatures close to 37°C, the diffusion of water through bacterial cell walls, and the availability of nutrients. Alternatively, spores can be killed at extremely high temperatures or aggressive sterilization processes without the need for activating spore germination.

Summary
Overall, sterilization is any process that removes, kills, or deactivates all forms of life. Sterilization for medical devices and products is critical for ensuring patient safety during product use. Three primary microbe types require appropriate sterilization (elimination) before medical devices are used: bacteria, fungi (yeast and mold), and viruses. Out of these three microbial types, spore forms of bacteria are the most difficult to kill. Spore forms of bacteria are hard to destroy because bacterial endospores can survive temperatures up to 150°C and as low as near absolute zero. Further, endospores are resistant to chemical agents (including alcohol), ultraviolet radiation, extreme pH gradients, drought, and nutrition depletion. However, bacterial endospores can be destroyed with two methods. The first method is destruction at high temperatures in their spore state. The second method is through triggering endospore germination back to active bacterial cells that are easily killed through traditional means of sterilization. All in all, ensure you choose a contract testing organization that can provide appropriate sterilization validations for your product needs.
Ethide Labs is a contract testing organization specializing in Sterilization Validations & Sterility Testing. Ethide Labs also offers EO Residual Testing, Microbiology Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, Bioburden Testing, Package Integrity Testing & Environmental Monitoring services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
Cornell Department of Microbiology. Bacterial Endospores. Cornell University. 2021.
International Organization for Standardization. Sterilization of health care products- Moist heat- Part 1: Requirements for the development, validation, and routine control of a sterilization process for medical devices. Geneva (Switzerland): ISO; 2006. (ISO 17665-1:2006/(R)2016).
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
Marina Basta and Pavan Annamaraju. Bacterial Spores. StatPearls. 2021.
United States Pharmacopeial Convention. <1111> Microbiological Examination Of Nonsterile Products: Acceptance Criteria For Pharmaceutical Preparations And Substances For Pharmaceutical Use. Rockville, MD, USA. 2021. (USPC <1111>).
United States Pharmacopeial Convention. <1115> Bioburden Control of Non-Sterile Drug Substances and Products. Rockville, MD, USA. 2021. (USPC <1115>).
United States Pharmacopeial Convention. <1116> Microbiological Control & Monitoring of Aseptic Processing Environments. Rockville, MD, USA. 2021. (USPC <1116>).
United States Pharmacopeial Convention. <1211> Sterility Assurance. Rockville, MD, USA. 2021. (USPC <1211>).
Share this in your social networks


