What is electron beam sterilization?
What is sterilization, and why is it essential for medical devices?
Sterilization keeps patients safe from toxins and microbial illnesses when therapies or devices are consumed or used. Sterilization is any process that removes, kills, or deactivates all microbial life. Under the strictest definition of sterility, an item or product is sterile when there is the complete absence of viable microorganisms (bacteria, yeasts, viruses, and molds). Sterility is defined by regulatory acceptance criteria based on calculated contamination probability. An acceptable level of contamination risk for most items is the probability of a single contaminated product out of a million manufactured products. However, sterility criteria may be more stringent or lax depending upon the intended use of the medical device or product.
What are the top medical device sterilization methods?
There are seven primary methods for medical device sterilization. These methods are steam sterilization, radiation sterilization, dry heat sterilization, sterilization by filtration, gas sterilization (such as ethylene oxide sterilization), vapor sterilization, and liquid sterilization. Of these sterilization methods, gas sterilization, vapor sterilization, and liquid sterilization techniques are chemical sterilization processes. As you might expect, steam and dry heat sterilization are thermal sterilization processes.
What is radiation sterilization (sterilization by radiation)?
Sterilization by radiation is a non-thermal sterilization method that functions by destroying any microorganisms in a product with gamma radiation, beta particles (electron beam), x-ray, or ultraviolet (UV) light. Other than sterile filtration, sterilization by radiation is the only other sterilization method that doesn’t rely on elevated temperature to sterilize. Sterilization by radiation is an excellent alternative for products that cannot be sterilized with heat or chemicals.
What is electron beam (e-beam) sterilization?
Electron beam (e-beam) sterilization has been gaining popularity for medical devices since its release in the 1960s due to its safety, speed, and negligible emissions. Electron beam sterilization is a form of radiation sterilization that utilizes beta particles to inactivate microbes. Electron beam systems scan medical devices with focused electrons (beta particles) to sterilize them. E-beam delivers higher dosage rates with less penetration compared to gamma radiation.
Some benefits of electron beam sterilization are:
- E-beam sterilization is an internationally accepted and FDA-approved process.
- E-beam has a high dosing rate and sterility assurance level (SAL), allowing for immediate release (no batch-to-batch sterility testing is needed after sterile processing)
- E-beam can penetrate a variety of materials, including foils.
- E-beam processes allow for temperature control during irradiation.
- A well-controlled dose range can be achieved with e-beam sterilization.
- E-beam sterilization is cost-effective.
- E-beam sterilization is a fast process (a minute in small lots), allowing near-immediate access to fully sterilized products.
- The speed of e-beam dosing protects the product’s material properties, prevents polymer degradation, and causes no damage to sterile seals on product packaging.
- E-beam has a minimal atmospheric effect and only releases a slight amount of ozone.
- E-beam sterilization doesn’t require a localized radioactive source.
Some of the drawbacks to electron beam sterilization are:
- E-beam sterilization institution construction is expensive.
- There are few e-beam radiation sterilization centers available for bulk sterilization.
- E-beam sterilization is less penetrative than radiation sterilization utilizing gamma.
- A concern when sterilizing finished products or active pharmaceutical ingredients (APIs) with beta particles is the risk of radiolytic byproduct formation (e.g., *OH) that could cause damage to the raw material, API, or product packaging system.

What items can be sterilized by electron beam?
Standard devices and materials sterilized with electron beam are plastics, heat-labile materials, glass, and powders. E-beam irradiation can also be used for tissue materials like aortas, bone, cardiovascular valves, and hydrogels. Radiation damages the nucleoproteins of microorganisms, and thus, electron beam sterilization is not recommended for biologics.
How is electron beam sterilization performed?
Simply speaking, e-beam sterilization is performed by exposing a product to beta particles. Beta particles are not electromagnetic and less penetrative than ionizing gamma radiation. However, most e-beam sterilization requires an electron accelerator, a rare and specialized machine. In electron accelerator systems, electrons are accelerated to near the speed of light and at high electron concentrations. Electron absorption by the product undergoing sterilization destroys the DNA of any live microbes. The mechanism of microbial DNA destruction is called DNA chain cleavage. DNA chain cleavage occurs by changing the DNA’s chemical and molecular bonds.
E-beam sterilization cycle characteristics depend on accelerated energy and adsorbed dose. The absorbed dose is the amount of interaction between the e-beam and the product that will be sterilized. The adsorbed dose is often defined as the absorbed energy per unit mass (Jewels per kilogram). Absorbed dose and absorption depth depend on the acceleration energy. Further, the microbial survival fraction is inversely proportional to the absorbed dose. Thus, the higher the absorbed dose, the smaller the fraction of surviving microbes.
Electron beam radiation effectiveness is dependent on the radiation dosage and time exposure. A 12-D sterilization overkill approach is often used for electron beam sterilization. The 12-D stands for providing a radiation dose sufficient to produce a 12-log reduction in the D-value of the most resistant microbial spore. Note that D-value determination for radiation uses dosage rather than time. A typical D-value range for the most resistant bacterial spore (Bacillus pumulis) to radiation is 1.7 to 2.0 megarad (MRad). A 12-D radiation dosage of 25 mRad is common, as this dosage is greater than 12-fold the D value of B. pumulis. However, the total radiation dose for e-beam sterilized products with tissue materials is in the range of 2 Mrad.
When a product goes through an e-beam sterilization conveyor (see Figure 1 below), the product moves under the e-beam at a set speed to obtain the desired electron dosage for the sterilization process. The total radiation dosage is distributed throughout the conveyor system so that there is no change in dose over time. An e-beam sterilization process can be adjusted by altering conveyor speed, which impacts the applied dose of the beam current.
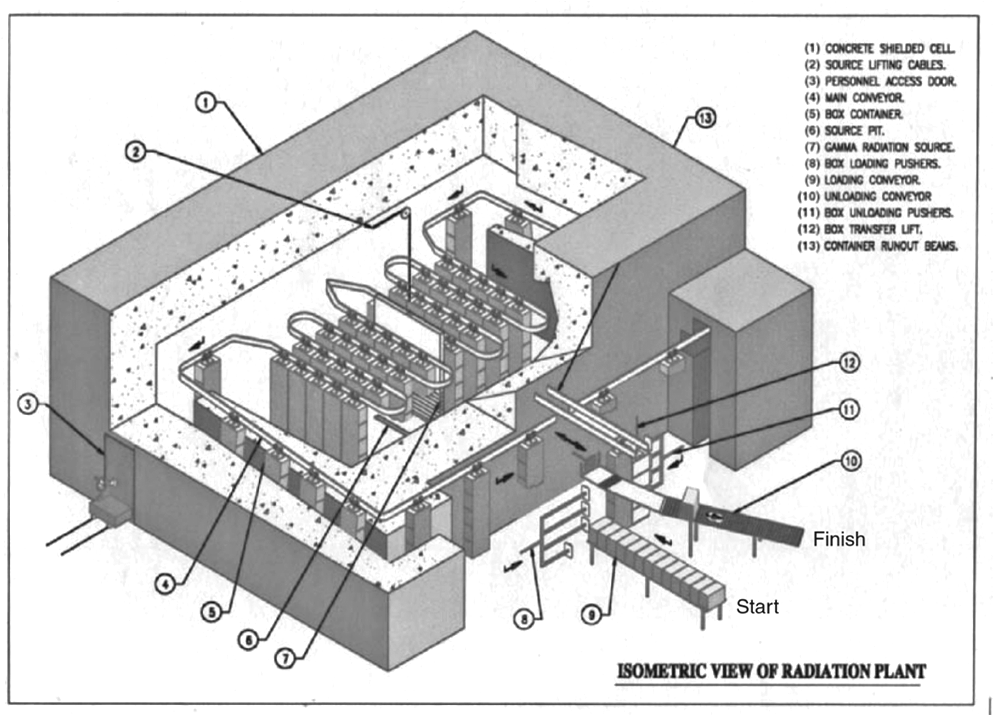
Factors that affect electron beam sterilization:
- D value of the biological indicator or bioburden level of the item undergoing sterilization
- Radiation strength (accelerated energy)
- Radiation dose rate (absorbed dose)
- Conveyor speed
Summary
Overall, medical devices, products, and therapies must be sterile. Sterilization is any process that removes, kills, or deactivates microbes. Electron beam sterilization is a form of radiation sterilization that utilizes beta particles to inactivate microbes. An alternative to heat sterilization, an electron beam is commonly used to sterilize tissue materials, plastics, heat-labile materials, glass, and powders. While an effective and rapid sterilization method, industrial electron beam sterilization is harder to find than industrial-level steam and ethylene oxide sterilization offerings. All in all, ensure you choose a contract testing organization that can provide appropriate sterility testing for your product needs.
Ethide Labs is a contract testing organization that specializes in Sterilization Validations and Sterility Testing. Ethide Labs also offers Bioburden Testing, Microbiology Testing, Bacterial Endotoxin Testing, Ethylene Oxide Residual Testing, Cytotoxicity Testing, Environmental Monitoring & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
Michael J. Akers. Sterile Drug Products Formulation, Packaging, Manufacture, and Quality. Drugs and the Pharmaceutical Sciences. Informa Healthcare. 2010.
Mine Silindir and A. Yekta Ozer. Sterilization Methods and the Comparison of E-Beam Sterilization with Gamma Radiation Sterilization. FABAD J. Pharm. Sci., 34, 43-53. 2009.
United States Pharmacopeial Convention. <1211> Sterility Assurance. Rockville, MD, USA. 2021. (USPC <1211>).
United States Pharmacopeial Convention. <1229> Sterilization of Compendial Articles. Rockville, MD, USA. 2021. (USPC <1229>).
United States Pharmacopeial Convention. <1229.10> Radiation Sterilization. Rockville, MD, USA. 2021. (USPC <1229.10>).
Share this in your social networks


