How Do You Select Biocompatibility Tests For Medical Devices & Implants?
What are medical devices?
Medical devices cover many non-pharmaceutical products used for disease treatment, monitoring, or prevention. For example, medical instrumentation (electronics), mechanical devices, and combination devices are all included in the definition of a medical device. Some combination devices have chemical, pharmaceutical, or biological components. Medical devices also cover implantable devices, devices used during surgical procedures, and non-implantable devices used at home by patients. Not all medical devices are equal. Medical devices in direct or indirect contact with the cardiovascular system or soft body tissues must undergo additional biocompatibility testing (ISO biocompatibility test 10993 requirements) compared to other medical devices. Thus, medical implants manufacturing and device manufacturing must account for biocompatibility tests for medical devices.
Examples of medical devices in direct or indirect contact with vascular system or soft tissues:
- Solution administration sets
- Extension sets
- Transfer sets
- Blood administration sets
- Intravenous catheters
- Dialyzers and dialysis tubing
- Transfusion and infusion assemblies
- Intramuscular drug delivery catheters
What is biocompatibility?
For ISO biocompatibility test 10993, biocompatibility refers to the ability of products to remain biologically inert while in contact with the body.
What is biocompatibility testing?
Biocompatibility testing is relevant for drug containers, elastomeric closures, medical devices, and implants. The ISO biocompatibility test 10993 requirements are completed to identify any biologically reactive physical or chemical components of a medical device. Both inherent or acquired toxicity from medical devices can be identified through ISO biocompatibility test 10993 requirements.
Medical devices and implants are evaluated for:
- Sterility Tests (USP 71)
- Bacterial Endotoxins Test (USP 85) or Pyrogen Test (USP 151)
- In-Vitro Biological Reactivity Tests (USP 87) or In-Vivo Biological Reactivity Tests (USP 88)
- Medical Devices Bacterial Endotoxin and Pyrogen Tests (USP 161)
- Elastomeric Components Used in Injectable Pharmaceutical Packaging/Delivery Systems (USP 381)
- Plastic Packaging Systems and Their Materials of Construction (USP 661)
- Plastic Materials of Construction (USP 661.1)
- Plastic Packaging Systems for Pharmaceutical Use (USP 661.2)
Medical devices and implants may also be evaluated for:
- Sensitization
- Irritation or intracutaneous reactivity
- Acute systemic toxicity
- Implantation
- Subchronic toxicity
- Genotoxicity
- Hemocompatibility
- Chronic toxicity
- Carcinogenicity
- Reproductive or developmental toxicity
- Biodegradation
Devices that undergo USP 85 bacterial endotoxins testing do not require USP 151 pyrogen testing. Further, devices that undergo in-vitro biological reactivity testing (also known as cytotoxicity testing) will not require USP 88 in-vivo testing.
All plastics testing (i.e., USP 381, USP 661, USP 661.1, and USP 661.2) depends on the materials utilized in the medical device construction or packaging. There are no current USP guidelines for subchronic toxicity, genotoxicity, chronic toxicity, carcinogenicity, hemocompatibility, reproductive toxicity, or biodegradation testing. All other toxicity tests, such as sensitization testing, are covered in the USP chapters listed in Table 1.
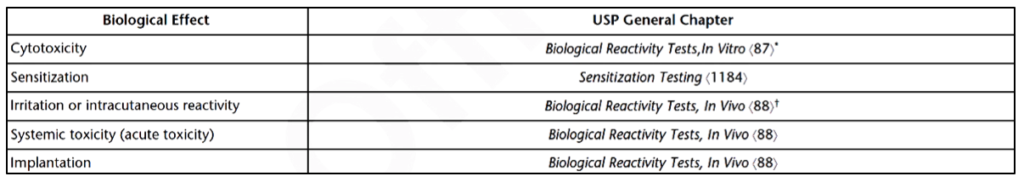
What do the required biocompatibility tests for a medical device or implant depend on?
- Similarity and uniqueness of the product relative to previously marketed products
- Extent and duration of the contact between the product and the patient
- Material composition of the product
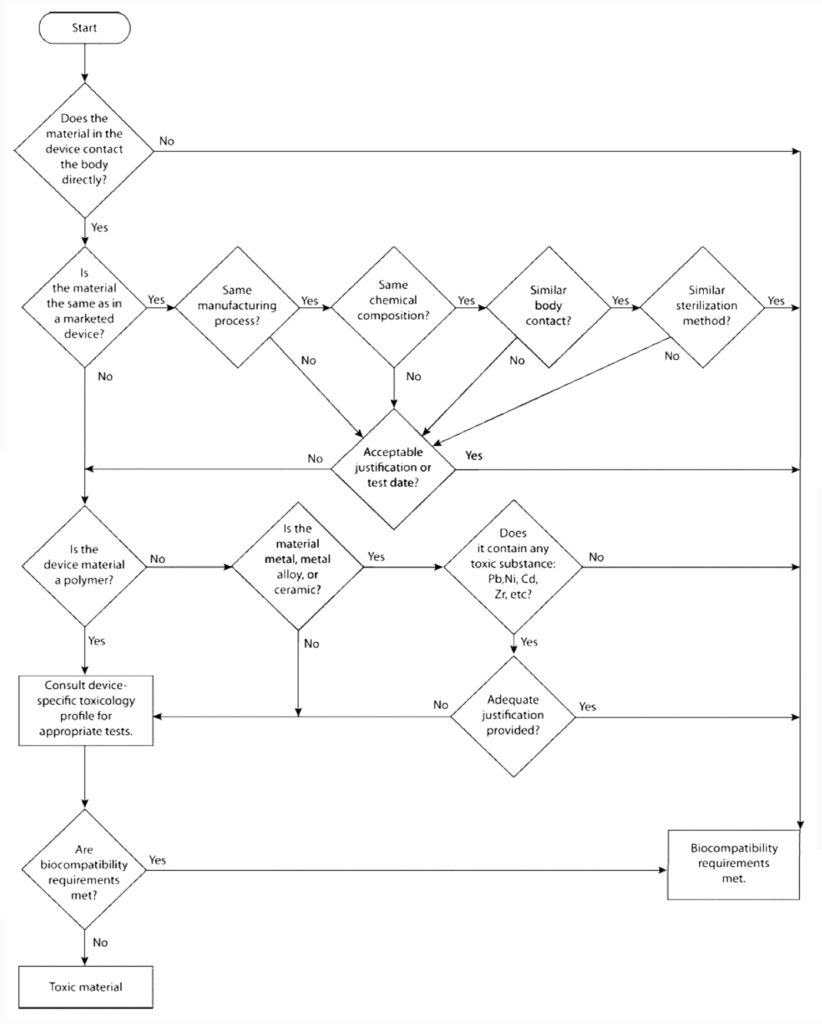
How do you compare your medical device or implant to previously marketed products?
United States Pharmacopeia created a biocompatibility decision flowchart (Figure 1) to support comparing new products with existing products on the market. If the new device is similar enough to an existing device, existing device data may be enough to prove the safety of the new device to be marketed. Devices using unique materials will always require additional toxicity tests.
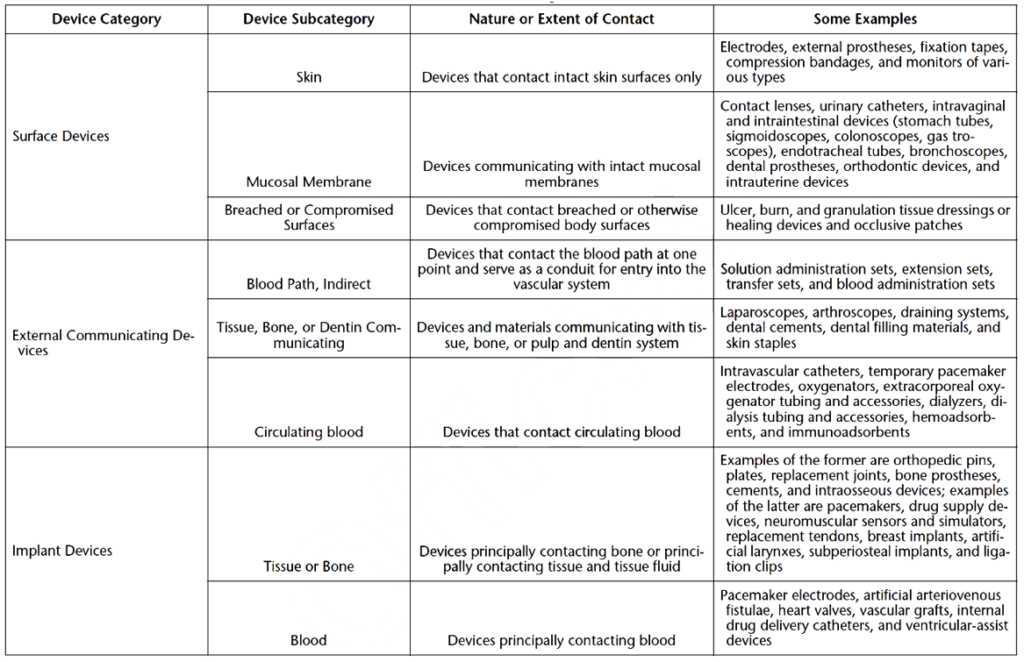
How are medical devices categorized for biocompatibility testing?
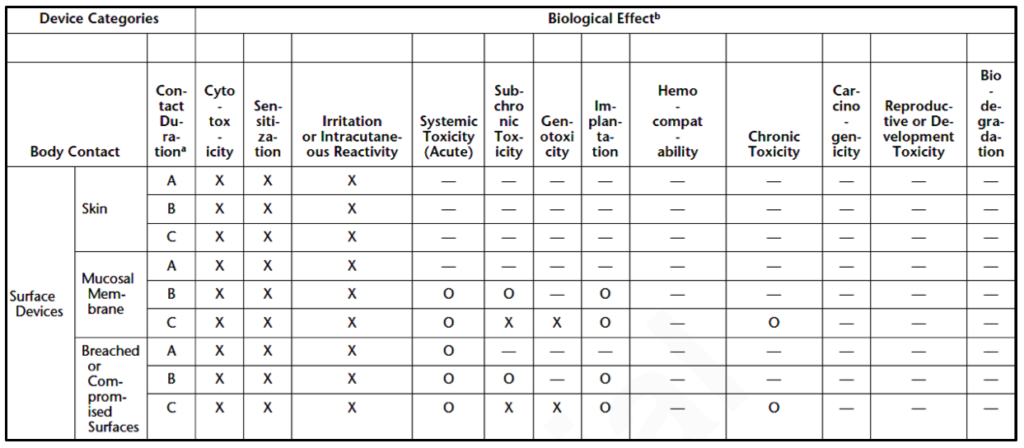
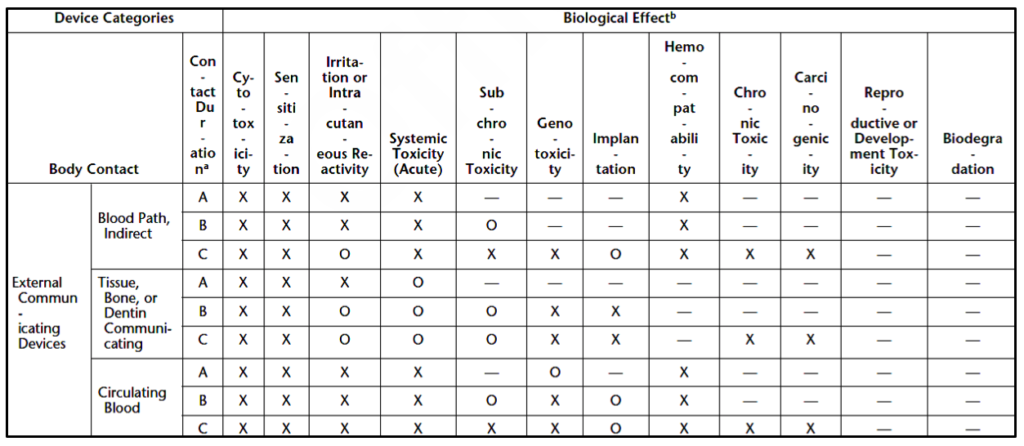
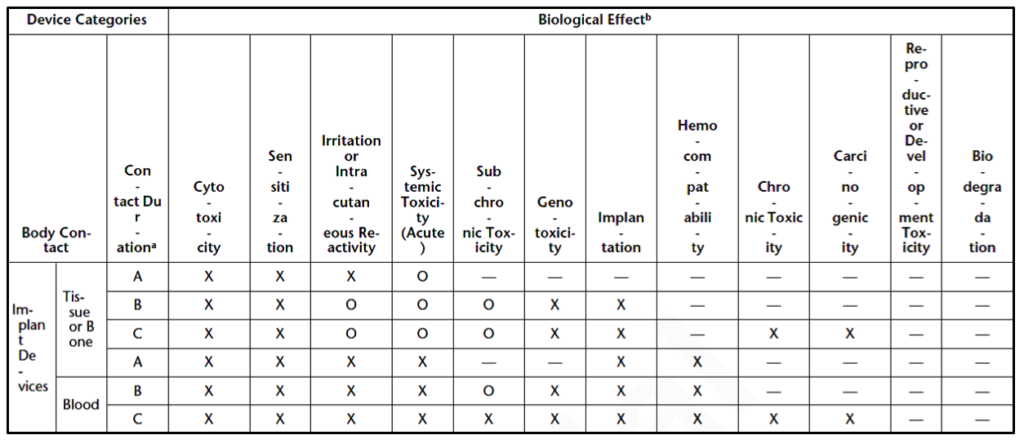
Medical devices are divided into three device categories for biocompatibility testing based on their intended use and the extent of their bodily contact. These device categories are surface devices, external communicating devices, and implant devices. Medical devices can be further subcategorized by the parts of the body they interact with, as shown in Table 2. These device categories and subcategories determine the necessary biocompatibility tests for each new medical device.
Which biocompatibility tests are necessary for your medical device?
The United States Pharmacopeia outlines three matrixes for biocompatibility test necessity based on the device categories specified above (i.e., surface devices, external communicating devices, and implant devices). Surface device biocompatibility tests are displayed in Table 3. Tests for external communicating devices are shown in Table 4, while tests for implant devices are displayed in Table 5. Each category of devices is subcategorized by the tissues the device contacts, and the duration of contact. Body contact duration is represented as (A) limited (less than 24 hours), (B) prolonged (24 hours to 30 days), or (C) permanent (more than 30 days) in Tables 3-5. As the amount of contact between the device and the body increases, the biocompatibility testing requirements increase. Similarly, biocompatibility testing requirements rise as the device’s bodily contact moves toward the circulatory system. Reproductive or developmental testing is only required for devices used by pregnant women or children.
The biological effects that are included in the matrix are:
- Cytotoxicity (aka biological reactivity)
- Sensitization
- Irritation or intracutaneous reactivity
- Systemic toxicity
- Subchronic toxicity
- Genotoxicity
- Implantation
- Hemocompatibility
- Chronic toxicity
- Carcinogenicity
- Reproductive or developmental toxicity
- Biodegradation
Summary
Overall, medical devices cover a vast range of nonpharmaceutical products used for disease treatment, monitoring, or prevention. Some combination devices have chemical, pharmaceutical, or biological components. Medical devices also cover implantable devices, devices used during surgical procedures, and non-implantable devices used at home by patients. Biocompatibility testing is relevant for drug containers, elastomeric closures, medical devices, and implants. The biocompatibility testing is completed to identify any biologically reactive physical or chemical components of a medical device. The biocompatibility tests required for a medical device or implant depend on the product’s similarity to previously marketed products, the extent and duration of contact between the product and the patient, and the product’s material composition.
The United States Pharmacopeia (USP) outlines three test selection matrixes for biocompatibility testing based on whether the device is a surface device, external communicating device, or implant device. Each device type is subcategorized by the tissues the device contacts and the duration of tissue contact. Body contact duration is represented as (A) limited (less than 24 hours), (B) prolonged (24 hours to 30 days), or (C) permanent (more than 30 days). As the amount of contact between the device and the body increases, the biocompatibility testing requirements increase. Similarly, biocompatibility testing requirements rise as the device’s bodily contact moves toward the circulatory system. Reproductive or developmental testing is only required for devices used in pregnant women and children. All in all, make sure you choose a contract testing organization that can support you with appropriate biocompatibility testing for your medical device, implant, or product.
Ethide Labs is a contract testing organization that specializes in various biocompatibility and toxicity tests for medical devices, implants, and other products. Ethide Labs also offers Microbiology Testing, Bioburden Testing, Cytotoxicity Testing, Ethylene Oxide Residual Testing, Bacterial Endotoxin Testing, Sterility Testing, Environmental Monitoring & Package Integrity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Food & Drug Administration. How to Determine if Your Product is a Medical Device. United States FDA Article. 2019.
United States Pharmacopeial Convention. <1031> The Biocompatibility Of Materials Used In Drug Containers, Medical Devices, And Implants. Rockville, MD, USA. 2021. (USPC <1031>).
Share this in your social networks


