Special Considerations For Bacterial Endotoxin Tests For Medical Devices
How Does Bacterial Endotoxin Testing For Medical Devices Differ From Other Products?
Bacterial endotoxin tests (BETs) follow the Limulus Amoebocyte Lysate (LAL) testing procedures outlined in USP 85 (see Bacterial Endotoxin Testing For Medical Devices). However, for medical device BETs, a medical device’s elution is used as the product sample. In liquid medical devices, elution is not necessary for endotoxin testing.
Standard extraction of the medical device tested is performed by soaking the device or flushing the device’s fluid pathway with extraction fluid that has been heated to body temperature. The entire device or all components of the device claimed to be nonpyrogenic, including fluid pathways, must contact the extraction fluid during elution. The extraction fluid is kept in contact with the device for at least an hour. Extracts from individual medical devices are then pooled for endotoxin testing. Details on how product families are evaluated via BETs, endotoxin limits, and medical device product suitability for BET are given below.
Product Families
Product families for BETs are defined by common materials of construction and shared components. The choice of family members is justified and documented. If a product family contains many sizes, the device with the largest surface area is chosen as the representative member of the family to be used in testing. When it comes to kits, each type of device must have its own product endotoxin limit and must be tested and evaluated individually. Additional details on medical device items for testing are given in Table 1 of USP 161, reproduced below.
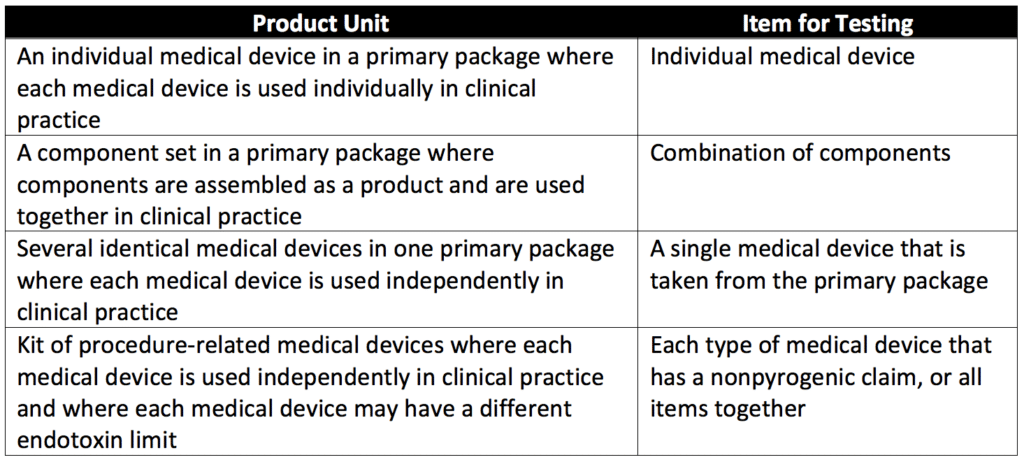
Endotoxin Limits
The endotoxin limit for a medical device:
- Not more than 20 USP Endotoxin Units (EU) per device
- Not more than 2.15 USP EU for devices in contact with cerebrospinal fluid
Note: Intraocular devices may also require a lower endotoxin limit.
The endotoxin limit for the extracting or rinsing solution (S) is:
S = (K × N)/V
Where:
K = amount of endotoxin allowed per device
(20 Endotoxin Units per device; 2.15 for intrathecal devices)
N = number of devices tested
V = total volume of the extract or rinse
Note: An extracting or rinsing solution is water or another solvent that does not interfere with the BET assay is used to extract and rinse the medical device. The rinse volume or extract volume depends on the size of the medical device being tested.
Suitability
Each product or product family must be tested to ensure that they are suitable and do not interfere with the BET. Medical device extracts or liquid medical devices are tested via a standard Gel-Clot Technique (see Bacterial Endotoxin Testing For Medical Devices). If needed, the pH of the test solutions will be adjusted with endotoxin-free solutions to pH 6.0–8.0. Sample solutions can be diluted in BET water or another validated solvent by a factor not exceeding the maximum valid dilution (MVD).
The MVD for medical devices is the following:
MVD = Endotoxin limit /λ
Where:
λ = sensitivity of the test method in Endotoxin Units/mL
Endotoxin limit = the endotoxin limit of the rinsing, extracting, or diluting solution in EU/mL
Note: Lambda (λ) is the test method sensitivity for BET gel-clot methods. For turbidimetric or chromogenic BETs, λ is equal to the lowest endotoxin concentration on the referenced standard curve.
Summary
Overall, BETs for medical devices differ from BETs for other products as a medical device’s elution is used as the product sample for endotoxin testing. The entire device or all components of the device that are claimed to be nonpyrogenic, including fluid pathways, must contact the extraction fluid for elution. When bacterial endotoxin testing is performed, the endotoxin limit for a medical device must not be more than 20 USP Endotoxin Units (EU) per device and not more than 2.15 USP EU for devices in contact with cerebrospinal fluid.
Keep in mind that medical device extractions will need to be assayed before endotoxin testing to ensure that the extractions do not interfere with the BET. We recommend consulting with a contract testing organization to ensure that you get the appropriate set of BETs for your medical devices and products.
Ethide Labs is a contract testing organization that specializes in Bacterial Endotoxin Testing. Ethide Labs also offers Bioburden Testing, Environmental Monitoring, Sterilization Validations, Microbiology Testing, EO Residual Testing, Package Integrity Testing & Cytotoxicity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <161> Medical Devices- Bacterial Endotoxin And Pyrogen Tests. Rockville, MD, USA. 2021. (USP <161>).
Share this in your social networks


