How To Perform Microbiology Testing For Nutritional & Dietary Supplements
What is microbiology testing for nutritional and dietary products, and why is it important?
Nonsterile nutritional and dietary products made through good manufacturing practices (GMP) require that “objectionable microorganisms” be absent from the products. Objectionable microorganisms are microbial contaminants that, depending on the microbial species, number of organisms, dosage form, intended use, and patient population, would adversely affect product safety. In other words, any microorganisms that are a potential health hazard to the user or could grow in the product to be potential health hazards to the user must be kept out of the product or removed. Microorganisms are also objectionable if they negatively affect product stability or could damage the integrity of the product’s container-closure system.
It is not intended that all nonsterile nutritional and dietary articles be tested for the absence of all hazardous microorganisms, only the microorganisms that are hazards to the individual product. This article will cover the tests for Staphylococcus aureus, Salmonella, Escherichia coli, and Clostridium. Staphylococcus aureus, Salmonella, Escherichia coli, and Clostridium are the most common microbial hazards to nutritional and dietary products.
How is microbiology testing for nutritional and dietary supplements performed?
For all tests below, 10 grams or 10 milliliters of the dietary or nutritional supplement are tested. Mix the 10 grams or 10 milliliters of the sample with 100 mL of Fluid Soybean–Casein Digest Medium (FSCD) for the initial incubation of the samples. If a plated medium is being tested, the surface of each plate is to be streaked with the loop in four directions to obtain a cross-crossed pattern of isolated colonies. Note that mixing is to be completed with gentle shaking so that the microbes are not mechanically damaged. For controls appropriate dilutions of Escherichia coli
(ATCC No. 8739), Staphylococcus aureus (ATCC1 No. 6538) and Salmonella typhimurium (ATCC No. 13311) will be used.
Test for absence of Staphylococcus aureus (S. aureus)
For S. aureus testing, FSCD broth is incubated at 30° to 35°C for 18 to 24 hours. A loopful of FSCD is then streaked onto the surface of one or more of Vogel–Johnson Agar Medium (VJ Agar), Mannitol–Salt–Agar Medium (MS-Agar), and Baird-Parker Agar Medium (BP Agar). Agar Petri plates are then covered, inverted, and incubated at 30° to 35C° for 24 to 48 hours. Then the agar plates are examined using the specifications in Table 1 below. If none of the agar plates contain colonies having the characteristics described in Table 1, the test samplings meet the requirement for the absence of Staphylococcus aureus. If S. aureus characteristic colonies are present, perform a coagulase test. To do a coagulase test, transfer the representative S. aureus colonies to separate tubes containing 0.5 milliliters (mL) of rabbit plasma, horse plasma, or any other mammalian plasma. Incubate the tubes in a water bath at 37°C. Then examine the tubes for coagulation after three hours of incubation and at suitable intervals up to 24 hours. The absence of a coagulase reaction indicates the absence of Staphylococcus aureus in the tested article. Make sure to have positive S. aureus and negative S. aureus controls for the coagulation experiment.
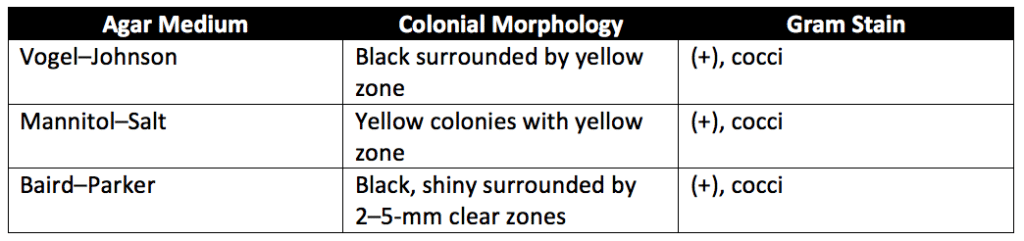
Test for absence of Salmonella species
FSCD broth is incubated at 30° to 35°C for 18 to 24 hours. After FSCD incubation, pipet a 1-mL aliquot into 10 mL of Rappaport Vassiliadis Salmonella Enrichment Broth. Then mix the samples and incubate them at 30° to 35°C for an additional 18 to 24 hours. Afterward, streak a loopful of each the FSCD incubation and Rappaport Vassiliadis Salmonella Enrichment Broth incubation media onto individual surfaces of one or more Brilliant Green Agar Medium (BG-Agar), Xylose–Lysine–Desoxycholate–Agar Medium (XLDC-Agar), and Hektoen Enteric Agar Medium (HE Agar). Next, cover, invert, and incubate agar Petri plates at 30° to 35C° for 24 to 48 hours. Then examine the agar plates using the specifications in Table 2 below. If none of the colonies have the characteristics of Salmonella, the test samples meet the requirement for the absence of Salmonella. If colonies with characteristics described in Table 2 are present, the colonies suspected to be Salmonella are transferred to a slant of Triple Sugar–Iron–Agar Medium (TSI) using an inoculating wire. The colonies are transferred by first streaking the surface of the slant with the colonies and then stabbing the wire well beneath the surface. After streaking, the slant is incubated at 30° to 35°C for 24 to 48 hours. If the tubes do not have red alkaline slants and yellow acid butts (with or without blackening of the butts from hydrogen sulfide production), the test specimens meet the requirement for the absence of Salmonella.
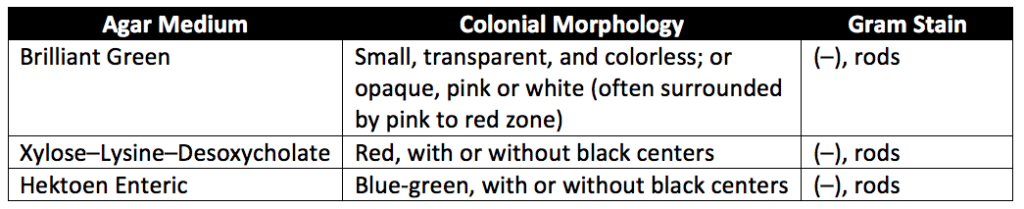
Test for absence of Escherichia coli (E. coli)
For E. coli testing, the FSCD broth is incubated at 30° to 35°C for 18 to 24 hours. After FSCD incubation, pipet a 1-mL aliquot of FSCD into a container containing 10 mL of MacConkey Broth. Then mix the MacConkey Broth samples and incubate them at 42° to 44°C for 24 to 48 hours. Then streak a loopful of each of the FSCD incubation and MacConkey Broth incubation media onto the surfaces of MacConkey Agar Medium (MC Agar) and incubate the agar at 30° to 35°C for 18 to 24 hours. Next, examine the inoculated MC Agar plates and interpret the results using the characteristics in Table 3 below. If none of the colonies have the E. coli characteristics described, the test specimens meet the requirements for the absence of Escherichia coli. Colonies suspected to be E.coli that show the characteristics described in Table 3 are transferred (individually) to the surface of a plate with Levine Eosin–Methylene Blue–Agar Medium (LEMB-Agar), using an inoculating loop. If many suspect colonies are transferred, divide the surface of each LEMB-Agar plate into quadrants. Each quadrant will be inoculated with a different, suspected E.coli colony. After inoculation, LEMB-Agar plates are covered, inverted, and incubated at 30° to 35°C for 24 to 48 hours. If none of the cultured colonies exhibit a characteristic metallic sheen under reflected light, and none exhibit a blue-black appearance under transmitted light, the test specimens meet the requirement for the absence of Escherichia coli.
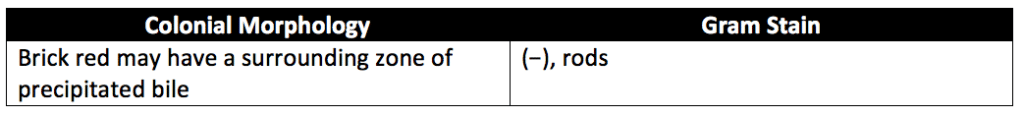
Test for absence of Clostridium species
Take two equal portions of the FSCD test preparation. Heat one to 80°C for 10 minutes, cool rapidly, then transfer 10 mL of each portion of FSCD (heated and unheated) into separate containers with 100 mL of Reinforced Medium for Clostridia. Next, incubate the containers under anaerobic conditions at 35° to 37°C for 48 hours. After incubation, subculture each specimen on Columbia Agar Medium (with gentamicin) and incubate under the same temperature and anaerobic conditions as before for 48 hours. Finally, examine the agar plates in reference to Table 4 below. If no growth of Clostridium microorganisms is detected, the test samples meet the requirement for the absence of Clostridium. If suspected Clostridium growth occurs, subculture each distinct colony on Columbia Agar Medium. Then separately incubate the agar plates in aerobic and anaerobic conditions at 35° to 37°C for 48 hours. The occurrence of only anaerobic growth of gram-positive bacteria (that also display an adverse catalase reaction) indicates the presence of Clostridium. To perform the catalase test, transfer individual colonies to glass slides. Then apply a drop of dilute hydrogen peroxide solution. The catalase test is negative if no gas bubbles evolve. If the test specimens exhibit a catalase reaction or can grow under aerobic conditions, the test specimens may meet the requirements for the absence of Clostridium.
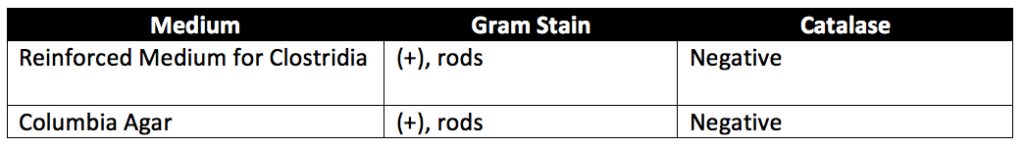
Retests
Retests may be performed to confirm a doubtful result. Traditional tests use 10 grams of a dietary or nutritional supplement. It is recommended that a retest be performed with a 25-gram sample of the nutritional or dietary supplement with the same procedures as described above and allowances for the larger specimen size.
Summary
Overall, dietary and nutritional supplements only need to be tested for the microorganisms that are hazardous for the user’s safety or the spoilage of the individual product. This article covers the tests for the absence of Staphylococcus aureus, Salmonella, Escherichia coli, and Clostridium. Staphylococcus aureus, Salmonella, Escherichia coli, and Clostridium are the most common microbial hazards to nutritional and dietary products. If you are looking to outsource the microbiology testing for your dietary and nutritional supplements, ensure you choose a contract testing organization that can support you with microbiology testing for your unique product needs.
Ethide Labs is a contract testing organization specializing in Microbiology Testing. Ethide Labs also offers EO Residual Testing, Cytotoxicity Testing, Bacterial Endotoxin Testing, Bioburden Testing, Package Integrity Testing, Sterilization Validations & Environmental Monitoring services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <2022> Microbiological Procedures For Absence Of Specified Microorganisms- Nutritional And Dietary Supplements. Rockville, MD, USA. 2021. (USPC <2022>).
Share this in your social networks


