How To Perform Environmental Monitoring For Sterility Assurance
What is environmental monitoring for medical products & medical devices?
Environmental monitoring is the tools and techniques used to observe an environment, characterize an environment’s quality, and ensure that an environment meets established acceptance criteria. Clean room environmental monitoring for medical products and medical devices covers the acceptance criteria (also known as sterility assurance levels) for the manufacturing environments throughout the entire lifecycle of the product, from raw materials to end-use or expiration. An excellent environmental monitoring program is an important for quality control. An environmental monitoring program is particularly critical for ensuring your medical device or product is devoid of microbes during various phases of manufacturing, packaging, transportation, and storage.
What is sterility assurance?
Sterility assurance is environmental monitoring that encompasses aseptic processing, post-aseptic fill terminal sterilization, and terminal sterilization. For each product, a certain sterility assurance level (SAL) must be met. In most cases, the sterility assurance level for a sterile product is a one in a million chance of product contamination. Non-sterile products (such as nasal or oral products) often have lower sterility assurance levels.
What are aseptic processes?
Aseptic processes are methods or procedures that are undertaken in a sterile environment. The aseptic sterile environment is maintained through specialized equipment that prevents microbial material from technicians, raw materials, or machinery from contaminating medical devices or products.
The terms aseptic and sterile are not synonymous. While both sterile and aseptic products will prevent microbial contamination following use, the processes by which microbial contamination is prevented are different. The term sterile means a complete absence of viable microorganisms or microbes that have the potential to reproduce. Thus, sterile products are often chemically or heat sterilized after being placed in their final packaging. The chemical or heat sterilization kills any microorganisms inside the products (obtained during manufacturing and packaging). This chemical or heat sterilization process after final product packaging is known as terminal sterilization. However, an aseptic process prevents contamination by the exclusion of microorganisms. Though the definitions for aseptic and sterile are not the same, sterile is used interchangeably with aseptic. Indeed, many products labeled as sterile are manufactured by aseptic processing rather than terminal sterilization. Aseptic processes can vary in complexity from comparatively simple filling-sealing to lengthy manufacturing sequences for complex items, such as medical devices.
Examples of medical products manufactured in aseptic environments:
- Pharmaceutical sterile products
- Bulk sterile drug substances
- Sterile intermediates
- Excipients
- Medical devices
- Biologics
What is terminal sterilization?
Terminally sterilized products are subjected to a final sterilization process that guarantees a quantifiable safety level, unlike products aseptically manufactured. Thus, in terms of microbial risk, terminally sterilized products are the lowest risk sterile medical products. Terminal sterilization most often occurs through a heat-steam method. However, chemical sterilization and irradiation (such as gamma or e-beam technologies) can also be used for terminal sterilization. Sterility assurance for terminally sterilized products is defined in terms of the probability of non-sterility (PNS). Terminal sterilization processes must achieve a PNSU of ≤ 1,000,000 (a probability of not more than one nonsterile unit in 1 million units produced.
What is post-aseptic processing terminal sterilization?
A post-aseptic processing terminal sterilization is a product manufactured through aseptic processes that are then terminally sterilized. Products manufactured with aseptic processing have control over the pre-sterilization bioburden, such that the subsequent terminal sterilization processing can be less harsh for the product or medical device. This could mean that products are exposed to lower temperatures, shorter cycle times, or reduced chemical exposure for the final sterilization process. For terminal sterilization of products that have been aseptically manufactured, the terminal sterilization cycle aim is to kill low bioburden organisms rather than pass biological indicators.
How do you perform environment monitoring for sterility assurance?
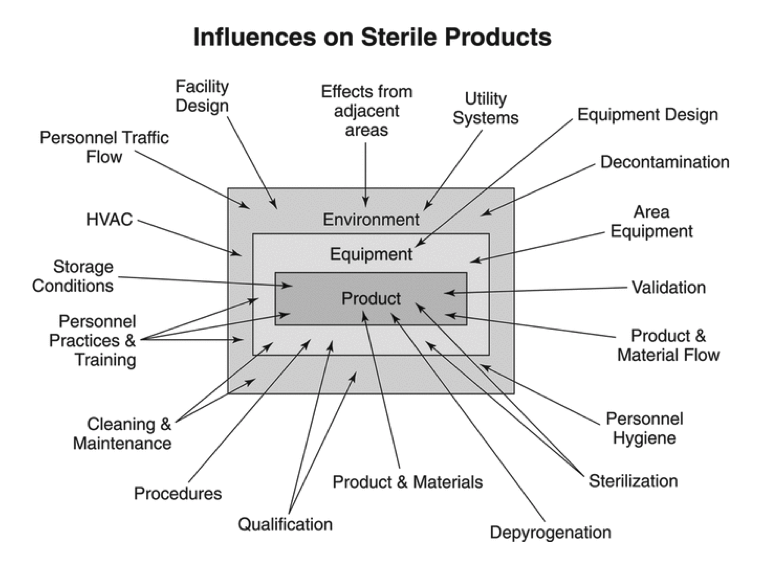
Clean room environmental monitoring for sterility assurance qualitatively assesses the effectiveness of a facility’s design and operational controls to provide sterile products. Environmental monitoring programs use qualitative measurements as it is difficult to control manufacturing environments to strict metric criteria. As detailed in Figure 1 below, many external factors influence product sterility. While environmental monitoring is important, it cannot substitute for good facility equipment, process design, and practices. Indeed, monitory only provides a snapshot of the actual environmental conditions occurring moment-to-moment in a manufacturing facility. Additionally, excessive environmental sampling can impair product safety and performance in critical anti-microbial areas. There are inherent limitations with all viable and non-viable monitoring forms in terms of sample size, sample location, and recovery capability in environmental monitoring programs. Sterility testing and aseptic process simulations also have their limits. However, viable monitoring, non-viable monitoring, aseptic process simulations, and sterility testing are all excellent methods for clean room environmental monitoring of medical devices and products. These techniques ensure that established performance criteria, according to the ISO classification of the room, are met.
Viable Monitoring
Viable monitoring involves microbial sampling techniques for detecting and estimating the level of culturable microorganisms in the air, on surfaces, and personnel.
Viable monitoring sampling methods include:
- Active air sampling
- Passive air sampling
- Viable particle counting using fluorescence technology
- A contact-plate sampling of surfaces, gloves, and gowns
- Swabbing of surfaces
- Personnel monitoring

Non-viable Monitoring
Non-viable monitoring measures the number and size of particulates (live or dead) present in the air with calibrated particle counters. Non-viable monitoring is used to initially classify the cleanroom in accordance with ISO 14644-1 and to assess routine manufacturing conditions periodically.
Aseptic Process Simulations
Process simulations evaluate the performance of an aseptic activity using a sterile growth medium. The sterile growth medium can be directly substituted for the product or added to it. Process simulations are fully representative of the current, new, or revised production processing conditions and activities. Aseptic process simulations are often performed prior to introducing a new or modified process component (new product, facility, equipment, personnel, etc.) to assess if the addition meets aseptic performance criteria.
Sterility Testing
Sterility testing is performed to determine if the material’s specifications are met. Lot-by-lot sterility testing for finished products must be completed unless parametric release testing is approved. The parametric release is a sterility assurance release program that demonstrates control of the sterilization process for consistent lot-by-lot sterility results. The parametric release is currently the most common mode of sterile product release.
Summary
Overall, the safety of sterile products requires that certain microbial specifications be met. Sterility ensures that medical products are devoid of living microorganisms capable of reproduction. Environmental viable monitoring, environmental non-viable monitoring, aseptic process simulations, and sterility testing are forms of microbiological analysis that have been historically employed as proof of “sterility.” Consider utilizing these techniques to ensure appropriate and consistent environmental monitoring for sterility of your medical product or medical device.
Ethide Labs is a contract testing organization that specializes in Sterilization Validations and Environmental Monitoring. Ethide Labs provides in-vitro cytotoxicity tests in-house and outsources in-vivo cytotoxicity work for toxicity testing of medical devices, products, and drugs. Ethide Labs also offers Bioburden Testing, Microbiology Testing, Bacterial Endotoxin Testing, EO Residual Testing, Package Integrity Testing & Cytotoxicity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <1211> Sterility Assurance. Rockville, MD, USA. 2021. (USPC <1211>).
Share this in your social networks


