What Are USP 61 Microbiological & Bioburden Testing Methods
What does USP 61 Cover?
USP 61 covers sample preparation, controls, and tests to quantify mesophilic bacteria and fungi. These testing techniques determine if a product meets quality specifications for bioburden testing and microbiology testing. These assays are not for items with viable microorganisms as active ingredients.
How are USP 61 product tests performed?
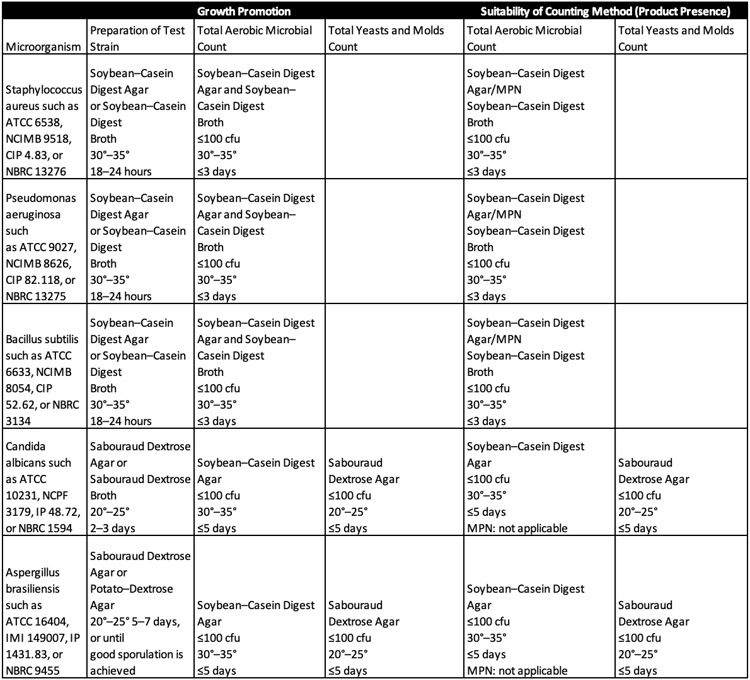
Test strains for each microorganism are prepared within five passages of the original seed lot. Each of the bacterial or fungal test strains is grown separately, as detailed in Table 1. A Buffered Sodium Chloride–Peptone Solution pH 7.0 or Phosphate Buffer Solution pH 7.2 is used to create test suspensions.
For most medical device specimens, 10 grams of product, 10 milliliters of product, or 10 milliliters of product extraction media to be examined is used for sample preparation. Exceptions to this are aerosolized products and transdermal patches. Active substances with small batch sizes test up to one percent of the total batch. During testing, each prepared sample and test control is exposed to 100 CFU of one of the microbes from Table 1. The volume of the microbial suspension should not exceed 1% of the volume of the diluted product when introduced.
Microbiological Examination Methods
Membrane filtration, pour-plate, surface-spread, and most-probable-number techniques are the most common methodologies used for bioburden testing. The most-probable-number method is typically the least accurate method for microbial counts unless the bioburden count is meager. Thus, most microbial counts are recommended for the membrane filtration method or the Plate-count Method. You can find brief descriptions for each of the bioburden testing methods mentioned below.
Membrane filtration method
First, appropriate dilutions of the product sample are prepared. Each of the sample dilutions is transferred to two membrane filters for rapid filtration. One of the membrane filters is transferred to the surface of a Soybean-Casein Digest Agar, and the other filter is transferred to the surface of a Sabouraud Dextrose Agar. Filters are washed between filtration of each sample dilution. The agar plates are then incubated between 3-7 days. Culture time and temperature are dependent upon the type of agar plate being incubated. After incubation, the Soybean-Casein Digest Agar is used to calculate TAMC and the number of CFUs per gram or milliliter of the product. The Sabouraud Dextrose Agar is used to calculate TYMC and the number of CFUs per gram or milliliter of the product.
Most-probable-number method
First, appropriate dilutions of the product sample are prepared. Tubes with the sample dilutions and controls are then incubated for 3 to 5 days at 30°C to 35°C. Following incubation, the tubes showing microbial growth are recorded, and the most probable number of microorganisms per gram or milliliter of the product sample is quantified.
Pour-plate method
As with the most-probable-number method, appropriate dilutions of the product sample being evaluated are prepared. Then at least two agar Petri dishes per sample dilution are made by pouring molten agar on top of the sample dilutions. The Petri dishes are incubated between 3-7 days. The timing of incubation and temperature of incubation depends upon the agar type. Plates showing the highest number of colonies of microorganisms following incubation that is less than 250 for total aerobic microbial count (TAMC) and 50 for total combined yeasts and molds count (TYMC), will be assessed for their colony-forming units (CFUs) per gram or per milliliter of the product tested.
Surface-spread method
The surface-spread method is nearly the same as the pour-plate method. The difference between the pour-plate method and surface spread method is that the agar is poured onto the sample dilution for the pour-plate method. In contrast, the sample dilution is spread on the surface of the solidified agar for the surface-spread method.
Results & Interpretation
When interpreting results, the total aerobic microbial count (TAMC) is equal to the number of colony-forming units (CFU), found with soybean-casein digest agar. Fungi colonies are counted as part of the TAMC. Similarly, the total combined yeasts and molds count (TYMC) is equal to the number of CFU found using Sabouraud Dextrose Agar. Colonies of bacteria detected are counted as part of TYMC. If TYMC goes over acceptance criteria due to bacterial growth, antibiotics may be used with the Sabouraud Dextrose Agar.
Acceptance criteria is as follows:
- Maximum acceptable Count = 20 for 101 CFU
- Maximum acceptable Count = 200 for 102 CFU
- Maximum acceptable Count = 2000 for 103 CFU
- And so on.
How does USP 61 relate to bioburden testing?
The “bio” in bioburden refers to live biological organisms, and the “burden” in bioburden refers to the concentration of the viable biological organisms. The higher the concentration of viable organisms on a device or product, the higher the burden is to kill those organisms. The USP 61 pharmacopeia methods outline how the microorganism testing for bioburden testing should be carried out. Specifically, USP 61 describes the preparation of test stains for aerobic microorganisms, product sample preparation, and numerous testing methodologies for bioburden testing and other microbial assays. When testing in-house or outsourcing your testing to a contract testing facility, it is important to make sure the USP 61 pharmacopeia methods are met for all bioburden testing. Additionally, bioburden testing should meet pharmacopeia methods from USP 60, USP 62, and USP 1111. A comparison between USP 60, USP 61, and USP 62 can be found here. USP 1111 limits and guidelines for bioburden
Ethide Labs is a contract testing organization that specializes in Bioburden Testing. Ethide Labs also offers Bacterial Endotoxin Testing, Environmental Monitoring, Sterilization Validations, Microbiology Testing, EO Residual Testing, Package Integrity Testing & Cytotoxicity Testing services for medical device companies and allied industries. Ethide is an ISO 13485 certified facility.
References
United States Pharmacopeial Convention. <61> Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests. Rockville, MD, USA. 2021. (USPC <61>).
Share this in your social networks